Scientist lays groundwork to find biological solutions to regenerate hearing
Sound waves created by rustling leaves or a baby’s cry, or any noise for that matter, travel to the inner ear, a maze of tubes and passages known as the labyrinth. This is where the magic happens: the sound waves are converted to electrical impulses that are sent to the brain, which in turn translates those impulses into sounds we recognize.
The fluids and delicate tissue of the inner ear, also known as the cochlea, are separated from circulating blood by the blood-labyrinth barrier. That its name evokes a sense of mystery is apt. Fairly little is known about the barrier, but its function is to protect the cochlea by keeping out harmful substances in the blood. That also means beneficial things like drugs and other therapies cannot reach the inner ear, notes Dr. Alain Dabdoub, a senior scientist at Sunnybrook Research Institute (SRI) and director of the Sonja N. Koerner Hearing Regeneration Laboratory at SRI.
His work is as fundamental as science can get, with a translational bent. Dabdoub’s lab is diving deep into the biology of the blood-labyrinth barrier. He wants to open up treatment options for hearing loss, a condition that affects 4.6 million Canadians and for which there is no cure.
Dabdoub is working on developing therapies for hearing loss, but the problem is getting them to where they need to go safely. “Right now, if you wanted to deliver something to the inner ear, you could either do an injection through the ‘round window,’ which causes trauma most of the time; or, through surgery, go into the cochlear part, to do delivery. Both are invasive,” says Dabdoub, who is also an associate professor in the departments of otolaryngology, and laboratory medicine and pathobiology at the University of Toronto.
Accessing the inner ear surgically is no delicate matter. “It’s not only the skull that’s the problem; it’s actually the bones surrounding the ear—they are the densest bones in the body,” he adds.
A safer, more elegant solution is to target therapeutics to the inner ear noninvasively using the circulatory system. With this goal in mind, he published a review paper in Science Translational Medicine on March 6, 2019 that summarizes the characteristics of the blood-labyrinth barrier.
Dabdoub and colleagues scoured the literature—citing more than 150 papers—to recap everything that’s been written about the blood-labyrinth barrier. In the article, they outline the cells that make up the barrier and conditions that affect how permeable it is, like inflammation and trauma. The researchers also compare the makeup of the barrier to another safeguard, the blood-brain barrier, which blocks both toxins and therapeutics in the blood from reaching the brain.
The next step, he says, is to use a technique called RNA [ribonucleic acid] sequencing, to identify the genes and receptor proteins on the surface of the cells that make up the barrier. Dabdoub aims to uncover receptors that can shuttle drugs in the bloodstream across the blockade. He notes that this strategy is already being used to deliver therapeutics packaged in nanomolecules across the blood-brain barrier. “The hypothesis is that some of these receptors are very similar, but not identical, to the ones expressed in the blood-brain barrier. Can we use what nanoparticles or carriers they’ve used to deliver across that barrier to the ear?” says Dabdoub.
Also not known is where the blood-brain barrier ends and the blood-labyrinth barrier begins. “We know at some point it changes. Where it changes and what the transition looks like—is it abrupt or not?—that’s something we’re interested in as well,” he says.
Once the biological mechanisms for crossing the blood-labyrinth barrier are understood better, therapies that might work include gene and stem cell therapies to regenerate sensory hair cells and auditory neurons. Hair cells turn sound waves into electrochemical signals. Auditory neurons take the signals to the brain. Both of these cell types deteriorate through aging, loud noises and medications that damage the inner ear, including chemotherapies and certain antibiotics.
Regenerative strategies that target the root cause of hearing loss are promising, but Dabdoub cautions patience. “This is an area that we think is ready for us to start learning more about being able to deliver [therapies] to the ear, but there’s more we need to discover.”
Dabdoub’s research into the blood-labyrinth barrier is supported by the Krembil Foundation. The lab is also supported by the Koerner Foundation and the Sunnybrook Hearing Regeneration Initiative.
Read text-only version of above infographic
Delivering therapies to the inner ear noninvasively
More than 4 million Canadians have hearing loss, caused by damage to the cells of the inner ear. Once these cells are lost, they are gone for good.
Sunnybrook Research Institute senior scientist Dr. Alain Dabdoub is working on biological solutions to regenerate hearing.
The only ways to deliver treatment to the inner ear, or cochlea, are by injection or surgery, which are invasive and cause trauma.
A safer option is to target therapies to the inner ear using the circulatory system, but there is an obstacle: the blood-labyrinth barrier.
What is the blood-labyrinth barrier?
It is made up of cells and proteins that keep toxins in the blood from reaching the inner ear. It also blocks drugs and other therapies.
Dabdoub published a paper summarizing what is known about this barrier. His lab is researching the properties of the barrier with the aim of bringing therapies to the inner ear noninvasively.
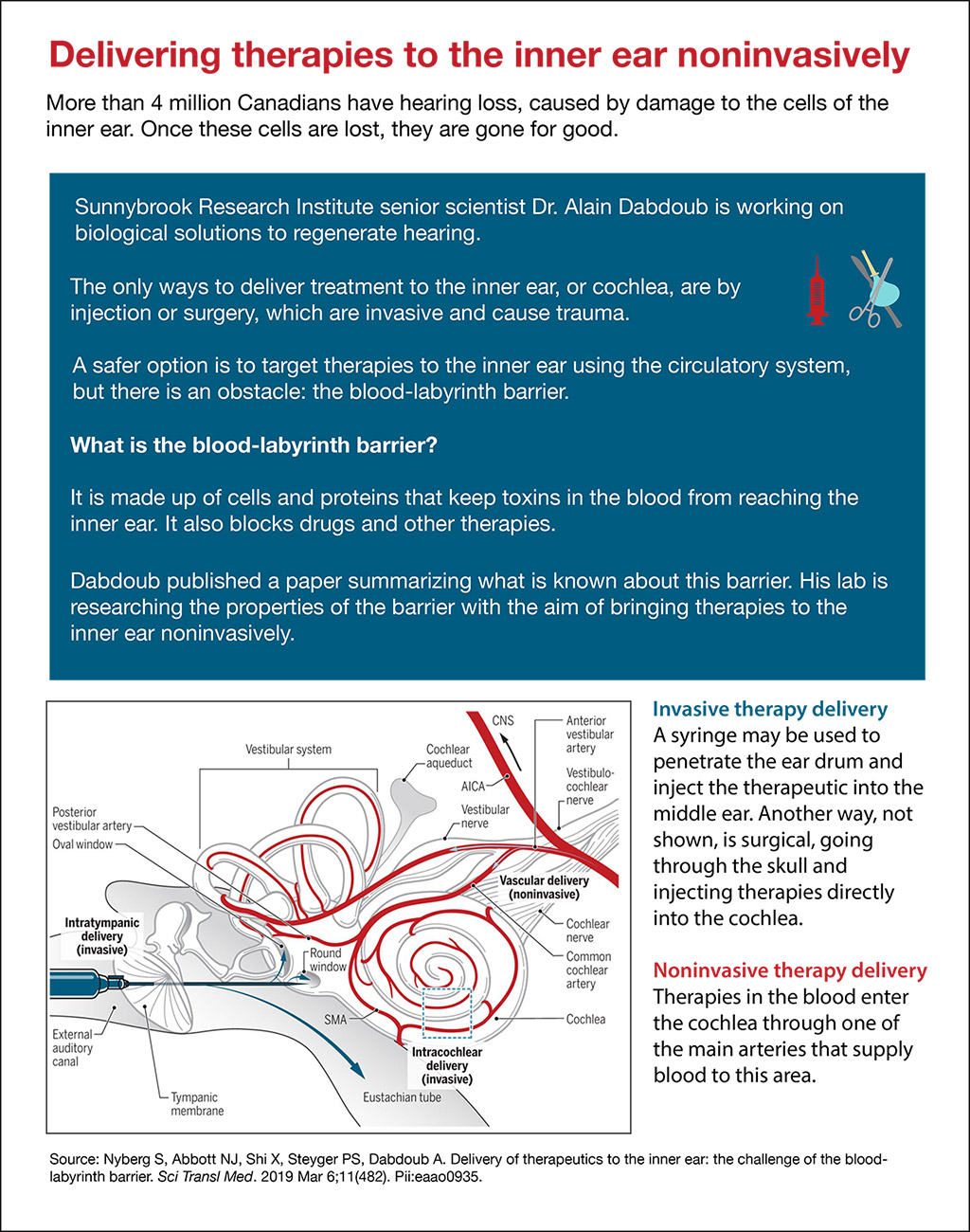
Invasive therapy delivery
A syringe may be used to penetrate the ear drum and inject the therapeutic into the middle ear. Another way, not shown, is surgical, going through the skull and injecting therapies directly into the cochlea.
Noninvasive therapy delivery
Therapies in the blood enter the cochlea through one of the main arteries that supply blood to this area.
Source: Nyberg S, Abbott NJ, Shi X, Steyger PS, Dabdoub A. Delivery of therapeutics to the inner ear: the challenge of the bloodlabyrinth barrier. Sci Transl Med. 2019 Mar 6;11(482). Pii:eaao0935.
Original article: Nyberg S, Abbott NJ, Shi X, Steyger PS, Dabdoub A. Delivery of therapeutics to the inner ear: the challenge of the blood-labyrinth barrier. Sci Transl Med. 2019 Mar 6;11(482). Pii:eaao0935. See Dr. Dabdoub’s SRI profile to read the full article.
In a nutshell
- Dr. Alain Dabdoub published a paper that summarizes what is known about the blood-labyrinth barrier, which keeps substances in the blood—both bad and good—out of the inner ear.
- His lab is researching the molecular properties of the barrier with the aim of bringing therapeutics to the inner ear noninvasively.
- One way of doing this is to identify receptors on the cells of the barrier that can transport gene or stem cell therapies to the inner ear via the circulation.