Innovative technologies need early-stage investment

Proof-of-principle funding is important to get medical products to market
A report by the Council of Canadian Academies, whose assessments inform public policy, shows that Canada punches above its weight in science and technology: despite having less than 0.5% of the global population, Canada produces nearly 5% of the world’s most frequently cited papers. When it comes to commercializing research, however, we could be doing much better.
Critics of this innovation gap point the finger at the risk aversion of Canada’s venture capitalists, who often require that technologies be well-developed and validated many times over before they consider investing in them. How, then, does a researcher with a promising idea get to that point?
This is where proof-of-principle (colloquially referred to as “PoP”) funding, comes in. These grants finance early-stage research and, critically, technology transfer activities that accelerate commercialization of intellectual property.
Scientists at Sunnybrook Research Institute (SRI) know that bringing the products of research to the marketplace is the only way patients can benefit from breakthroughs made in the lab. They are developing technologies to improve patient care and seizing opportunities to move them through the innovation pipeline.

Dr. Cari Whyne engineered bone tape so that surgeons can reattach fractured facial bones, as if fixing a broken vase.
Photo: shutterstock.com
Doctors trying to fix deformities of the face and skull during reconstruction surgery know all too well the drawbacks of using metal plates and screws to repair fractures of delicate facial bones. The hardware neither attaches well to thin bone, nor is suited to the complex geometry of the facial skeleton. Moreover, complications, including infection and prolonged pain, occur frequently.
Dr. Cari Whyne, a biomedical engineer and director of the Holland Bone and Joint Research Program at SRI, is working on an elegant solution: bone tape. As its name suggests, the technology adheres to bone and can reattach fractured pieces with enough stability until they heal.
“It works kind of like the way you’d expect a Band-Aid to work,” says Whyne. “[It’s] like duct tape for bones—to be able to tape multiple fragments together as if you’re fixing a broken vase, for example. It’s complicated, and there are a lot of parts that need to fit together. You need some flexibility to put that ‘puzzle’ back together in three dimensions.”
She is developing the bone tape with Dr. Jeff Fialkov, an associate scientist at SRI and a plastic surgeon at Sunnybrook, and researchers at the Institute of Biomaterials and Biomedical Engineering at the University of Toronto, where Whyne is also a professor.
Their preclinical studies show bone tape is effective, safe and easy to use. Over time, the technology, which is made of a polymer and ceramic, dissolves.
She was awarded a PoP grant from the Canadian Institutes of Health Research (CIHR) to optimize bone tape and make headway in commercializing it.
The researchers have filed a patent and done a workflow analysis to understand how the device would function in the operating room. Whyne notes they’ve made prototypes in the lab and are looking at ways of scaling up manufacturing. They will also do longer-term studies of how bone tape biodegrades.
She secured funding in the final competition of CIHR’s PoP program. As part of reforms made by the agency, the program, which gave out about $5 million annually, was subsumed within CIHR’s general “Project Scheme.” Critics argue shutting down the niche program, which covered non-research costs, including patenting and market studies, will hamper innovation.
Whyne says funding from the defunct program will be instrumental in proving the usefulness of bone tape, which will help attract an industry partner. Although commercialization is long and difficult, she is committed to seeing it through. “It’s the best way to get new technology into the operating room,” she says.
Dr. Simon Graham, a neuroimaging scientist at SRI, was awarded a PoP grant in 2011 to develop a technology for use during functional magnetic resonance imaging (fMRI). Conventional MRI visualizes anatomy; in contrast, fMRI shows physiological processes. It depicts changes in blood flow and blood oxygenation, which researchers use to map brain activity.
The tablet-based technology will enable people to do neuropsychological tests, used to assess thinking and normally done with pencil and paper, during fMRI scans.
The idea is to use the test results and the map of brain activity together to gain a comprehensive look at brain impairment early on in people with Alzheimer’s disease or mild cognitive impairment. “You combine the two and you might get improved sensitivity and specificity for characterizing a brain abnormality,” says Graham. The test would indicate cognitive impairment, and the brain activity map would pinpoint which regions are responsible for that impairment.
The MR environment is exacting. Patients must remain still during scans, which makes paper-and-pencil testing impossible. Using a tablet computer within the system has its own challenges, however. “You don’t want attractive magnetic forces causing the tablet to be pulled into the magnet and hurting the patient. You also don’t want the electronics of the tablet to interfere with the MRI signals and vice versa,” he says.
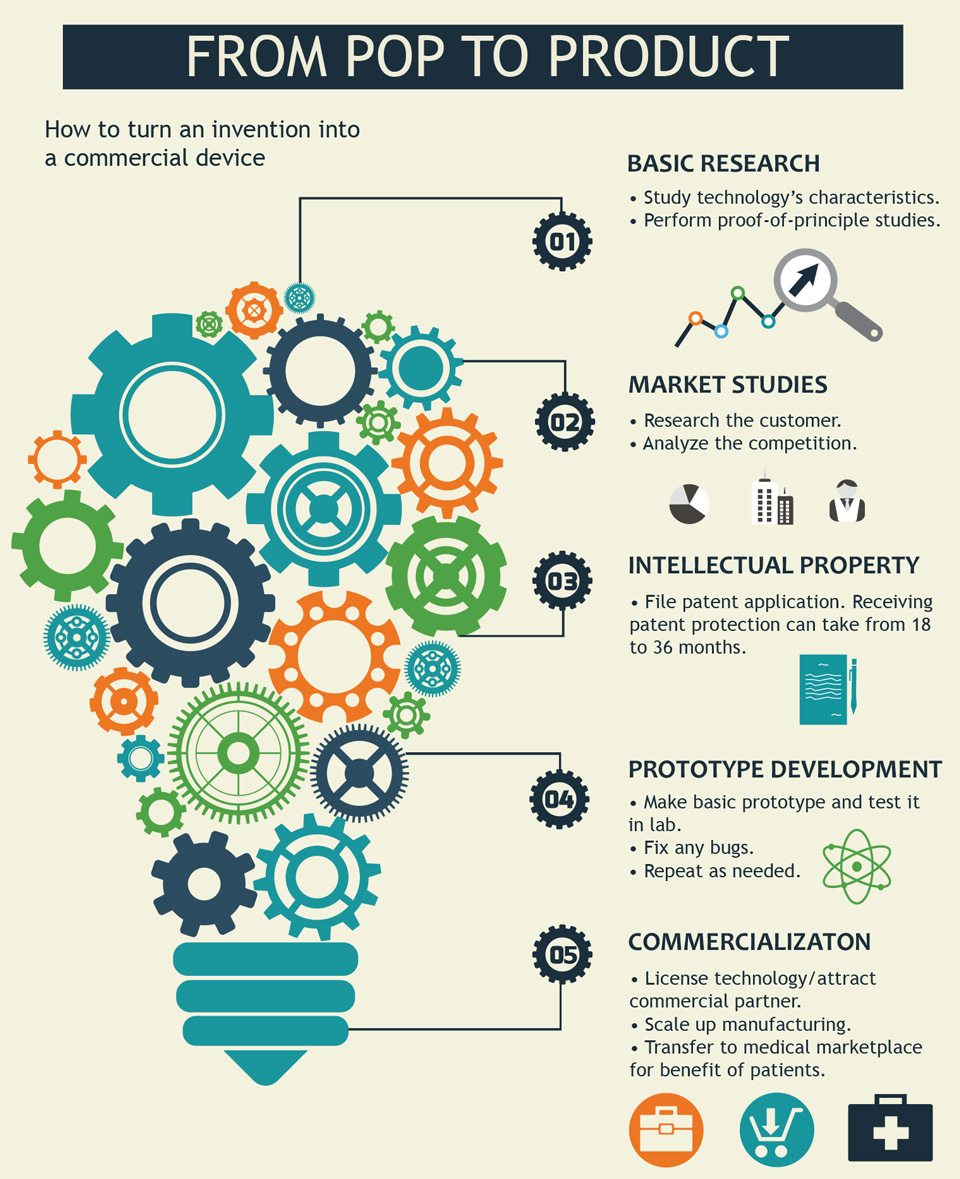
Read text-only version of above infographic
From PoP to Product
How to turn an invention into a commercial device:
- Basic Research
Study technology’s characteristics.
Perform proof-of-principle studies. - Market Studies
Research the customer.
Analyze the competition. - Intellectual Property
File patent application. Receiving patent protection can take from 18 to 36 months. - Prototype Development
Make basic prototype and test it in lab.
Fix any bugs.
Repeat as needed. - Commercialization
License technology/attract commercial partner.
Scale up manufacturing.
Transfer to medical marketplace for benefit of patients.
As a workaround, he separated the response aspect of the tablet (its writing surface) from the display component (its screen). Participants do tasks on a touch-sensitive surface that is fixed to a table. Through a mirror, they look at a projection screen that’s connected to a computer, which shows their responses and the test questions—a phone number they have to copy, for example.
A video camera inside the MRI system lets people see their hand and the writing instrument as they write. The visual feedback helps them make responses in the right spot, notes Graham.
He and his team have validated the technology on young, healthy adults. Next, they will use it to study healthy elderly adults and people with mild cognitive impairment.
Another exciting application is in neurosurgery. The technology has been used by patients who were awake during a part of surgery to remove a brain tumour. When combined with electrical stimulation of the cerebral cortex, the outer layer of the brain, it enables intraoperative brain mapping for safer, more effective surgery. In a well-documented case at St. Michael’s Hospital in Toronto, a surgeon used Graham’s technology to remove a seizure-causing tumour lodged deep within the brain of a 23-year-old woman.
The device is 10 years in the making. Graham says PoP money helped him to move forward with commercialization and technology development. Will the loss of this funding opportunity mean Canada’s performance on innovation—middling, according to the Conference Board of Canada, which ranked Canada ninth of 16th among peer countries on this measure—slip even further? Only time will tell, but much more is at stake than dollars and cents.
— Alisa Kim
Graham’s work on the tablet was supported by the Canada Foundation for Innovation (CFI), Canadian Cancer Society, CIHR, and Heart and Stroke Foundation of Canada.
Whyne’s work on bone tape is supported by CFI, CIHR, MaRS Innovation and the U.S. Department of Defense.



