Deep brain stimulation
Deep brain stimulation (DBS) is currently being used in the treatment of symptoms in movement disorders like Parkinson’s disease. Clinical trials are underway exploring the use of DBS for treatment-resistant forms of various disorders including post-traumatic stress disorder (PTSD), alcohol use disorder (AUD), obsessive-compulsive disorder (OCD) and major depressive disorder (MDD) at Sunnybrook’s Harquail Centre for Neuromodulation.
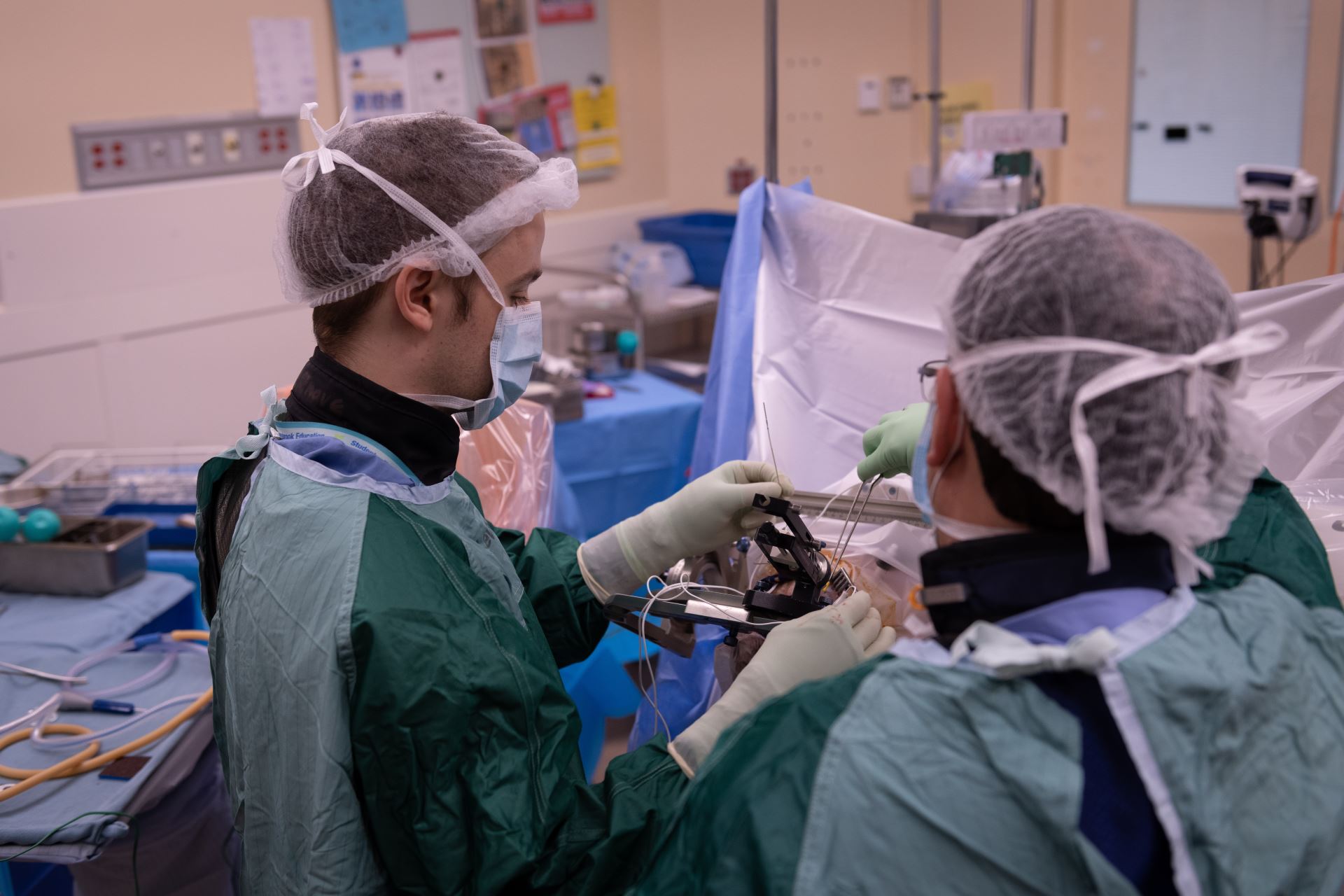
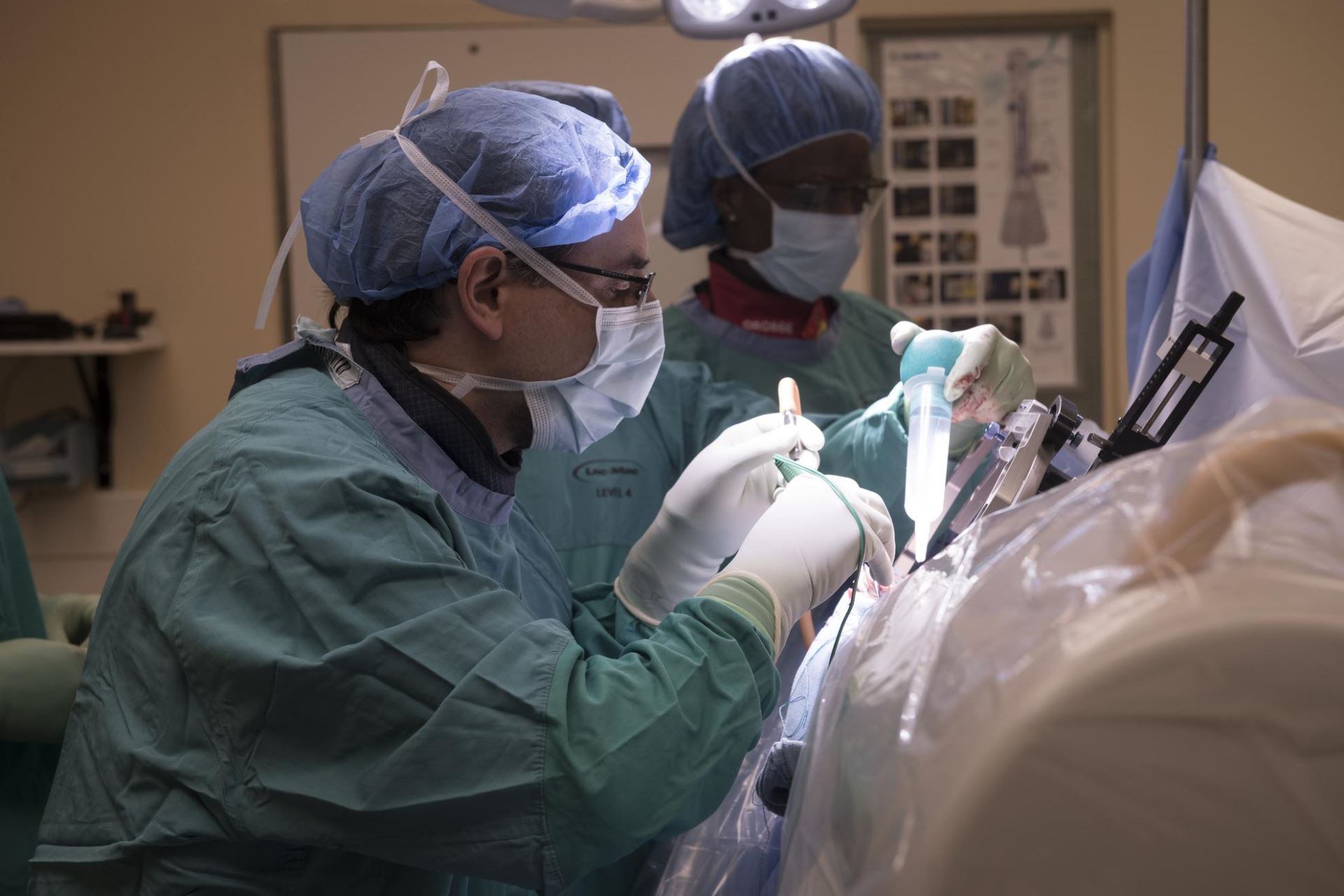
DBS is a neurosurgical procedure that directly impacts dysfunctioning brain circuits. It involves inserting thin electrodes into deep brain structures and electrically stimulating them in a controllable and ultimately reversible manner with a pacemaker-like device.
Learn more about deep brain stimulation
Sunnybrook researchers are conducting ground-breaking DBS trials. For the first time in North America our researchers have launched a study investigating the use of DBS for treatment-resistant AUD. They are also examining DBS in the treatment of hard-to-treat PTSD in a Canadian-first trial.
- December 20, 2022
- December 2, 2022
- July 8, 2020
- December 16, 2019
Frequently asked questions
What is DBS?
DBS involves implanting electrodes to influence abnormal activity in circuits of the brain. For over 25 years, DBS has been used to help treat Parkinson’s disease. More than 200,000 DBS surgeries have been performed worldwide, the majority, for movement disorders like Parkinson’s disease. It is also approved for the treatment of obsessive-compulsive disorder (OCD) in the United States, and is under active investigation for several neurological and psychiatric indications in Canada.
For the first time in North America, Sunnybrook researchers are investigating DBS as an experimental treatment for symptoms of treatment-resistant alcohol dependence. Our researchers are the first in Canada to conduct unique clinical trials exploring DBS for both treatment-resistant post-traumatic stress disorder (PTSD) and depression.
What equipment is involved in DBS?
DBS is like a pacemaker for the brain. Two electrodes travel through nickel-sized holes in the skull into precisely targeted brain regions. These electrodes are placed beneath the skin and connect to a battery just below the collarbone. The battery provides a small amount of electricity delivered through the tips of the electrodes in the targeted brain areas to interrupt brain circuits that are functioning abnormally.
What studies are investigating the use of DBS?
There are currently four active studies at Sunnybrook’s Harquail Centre for Neuromodulation investigating the use of DBS for the following disorders that are treatment-resistant, meaning they are not responsive to conventional therapies such as psychotherapy and medication. They include:
- DBS for alcohol use disorder (AUD)
- DBS for post-traumatic stress disorder (PTSD)
- DBS for major depressive disorder (MDD)
- DBS for obsessive compulsive disorder (OCD)
Is DBS a first-line treatment?
No. At this point, DBS is an experimental procedure that is being tested for safety for the treatment of severe psychiatric conditions.
To be included in the DBS trial for treatment-resistant AUD, PTSD, MDD, or OCD, a patient must have tried all other treatments including medication and psychotherapy. DBS is not intended to replace other treatments, but to add or complement other treatments in patients who are not improving with conventional treatments.
Current research suggests that there are similarities between the symptoms of PTSD and those of anxiety and depression.
Is DBS safe?
DBS is used in Canada and around the world to help alleviate symptoms of movement disorders like Parkinson’s disease and essential tremor. It is also being studied for possible treatment of psychiatric disorders such as OCD and depression.
For the first time in North America, Sunnybrook researchers are studying DBS in the treatment of patients diagnosed with alcohol use disorder who have not responded to conventional treatment.
Sunnybrook researchers are also the first in Canada to demonstrate that DBS is safe and potentially effective in treating in PTSD.
In a Canadian first, DBS is also being explored in treatment-resistant depression as a way of recording brain signals to find a ‘signature’ in mood.
What parts of the brain will be treated and why?
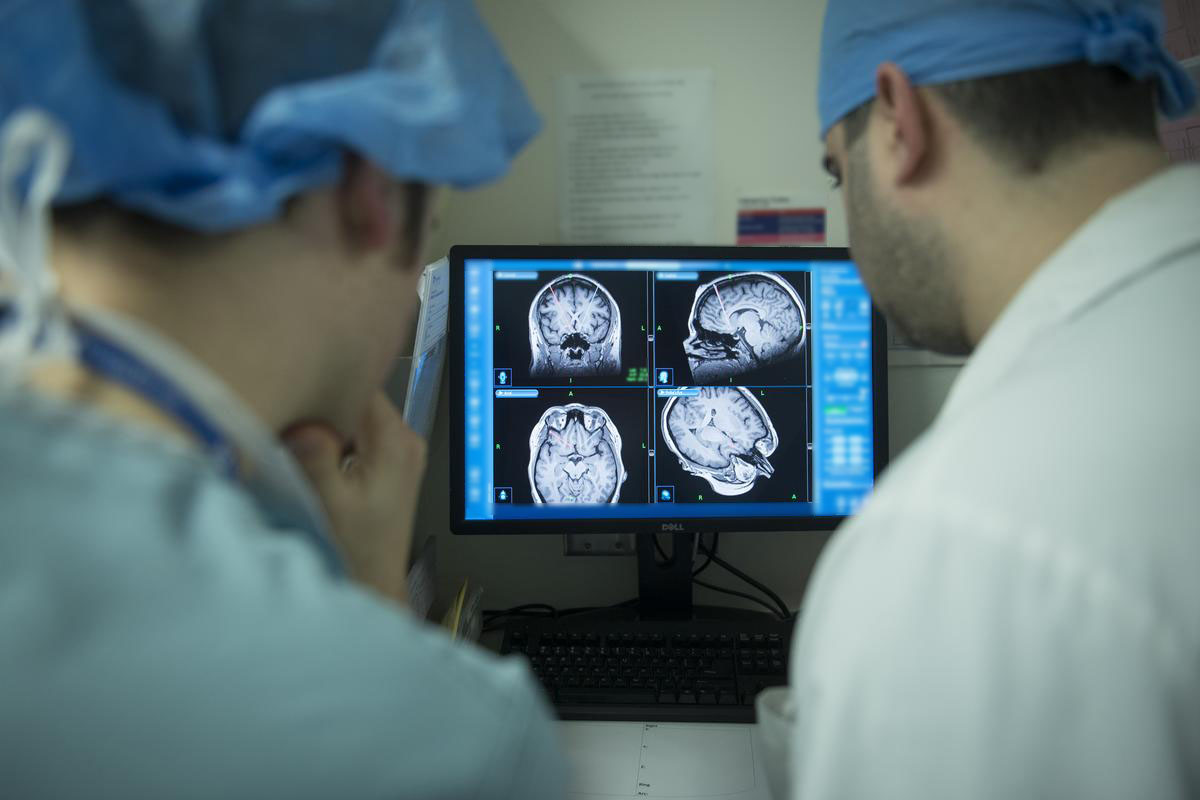
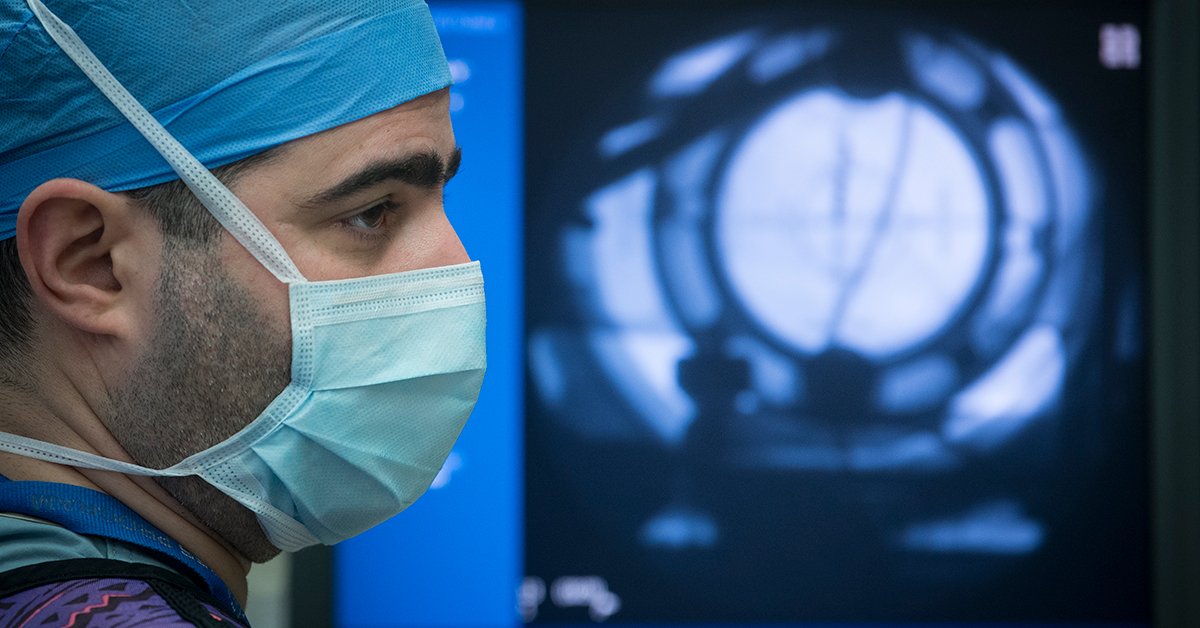
Different parts of the brain play a role in different disorders. In each trial, researchers will target circuits of the brain that may help regulate or control symptoms. The latest in brain imaging technology will help precisely locate the key regions as leading-edge, direct-to-brain therapies are being investigated.
How effective is DBS?
DBS for difficult to treat chronic alcohol dependence and treatment-resistant depression is currently being tested for safety as an experimental treatment at Sunnybrook. As a result, the effectiveness of DBS for AUD, severe depression, and PTSD is still under investigation. However, DBS is an established treatment for movement disorders such as essential tremor and Parkinson’s disease.
Who do I contact for more information?
A. For more information or to inquire about eligibility for the trial please contact Anusha Baskaran at harquailcentre@sunnybrook.ca or 416-480-6100 ext. 61650.
What are some other resources for PTSD?
Learn more about the trial from the researchers in Behind the Research: DBS: Pursuing new treatment options for PTSD
Canadian Mental Health Association: Post-Traumatic Stress Disorder (PTSD)
Veterans Affairs Canada: Post-Traumatic Stress Disorder (PTSD) and War-Related Stress
What are some other resources for alcohol use disorder?
What are some other resources for depression?
Information about depression can be found on Sunnybrook’s Department of Psychiatry webpage under Patient and Family Education Resources.
If you need help in an emergency, please call 911 or visit your local emergency department. If you’re feeling like you’re in crisis or need somebody to talk to, please know that help is also available through community resources:
- Find a local crisis resource at sunnybrook.ca/gethelp
- Crisis Services Canada
- Phone: 24-hour, toll-free 1-833-456-4566
- Text: 45645 (4:00 p.m. – midnight Eastern Time)
- Kids Help Phone
- Phone: 24-hour, toll-free, 1-800-668-6868
- Text: 686868 (24 hours, 7 days a week)