Hodgkin Lymphoma
Lead: Matthew Cheung
Date of last revision: June 25, 2020
Terms of use:
These guidelines are a statement of consensus of the OCC Hematology site group regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult the Guidelines is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient's care or treatment. Use of this site and any information on it is at your own risk.
-
Diagnosis and pathologic classification:
Hodgkin Lymphoma (HL) represents ~10-15 per cent of lymphoma presentations. It has a bimodal distribution, occurring more commonly adolescents/young-adults or in adults over 50. The 2017 WHO classification of Haematopoietic and lymphoid tumors a number of distinct diagnostic categories including [Swerdlow]: - Classical HL
- Nodular sclerosis
- Mixed cellularity
- Lymphocyte rich
- Lymphocyte depleted
- Nodular lymphocyte predominant
-
Baseline testing:
- Full history and physical including performance status/frailty index and documentation of B symptoms.
- CBC, Albumin, LDH, ESR, LFTs (Bilirubin, ALT, ALP), creatinine
- Hepatitis B surface antigen, Hepatitis B core antibody, Hepatitis C antibody and HIV serology and Covid-19 testing
- TB skin test
- CT head, neck, chest abdomen & pelvis
- FDG-PET/CT
- Bone marrow can be deferred if PET/CT completed at baseline, unless warranted to investigate unexplained cytopenias or to investigate uncertain results after PET/CT.
- MUGA scan or 2D echo (Age >60 or with cardiovascular risk factors)
- PFTs (Age >60, smoking history, or pre-existing lung disease)
- Pathology review if not reported at Sunnybrook or UHN
- Discuss sperm banking/fertility preservation
-
Staging and prognostic factors:
Patients should be staged according to the Ann Arbor staging system:
StageSymptoms1 Single lymph node region (1) or single localized extranodal site (1E). 2 Two or more lymph node regions, same side of the diaphragm (2) or local extranodal involvement in two or more regions, same side of the diaphragm (2E). 3 Lymph node regions on both sides of the diaphragm (3) which may be accompanied by local extranodal extension (3E). 4 Diffuse involvement of one or more extranodal organs or sites.
A = No B symptoms
B = presence of at least one of these:
- unexplained weight loss > 10 per cent baseline during 6 months prior to staging
- recurrent unexplained fever > 38oC
- recurrent night sweats
Prognosis for limited stage disease – defined according to the GHSG criteria:
- Presence of a large mediastinal mass (LMM), measured by means of a chest X-ray image; the mediastinal mass is considered large if it measures at least one third of the transverse diameter of the thorax.
- Extranodal disease, i.e. any tumor spread involving other tissues than those of the lymph nodes, spleen, thymus, Waldeyer’s tonsillar ring, appendix and Peyer’s patches.
- High erythrocyte sedimentation rate (ESR) of 50mm/h if A-symptoms are present, and 30mm/h if B-symptoms are present.
If none of the above are present, than the patient is considered limited stage – favourable. If any of the above are present, than the patients is considered limited stage – unfavourable.
If patient has stage IIB disease AND extranodal disease and/or LMM, then treat as advanced stage disease.
Prognosis for advanced stage disease – defined according to the Hasenclever IPS.
Age >45 years Gender Male Stage IV Hemoglobin <105 g/L Albumin, serum <40 g/L WBC >15.0 x 109/L Lymphocytes count <0.6 x 109/L or percent <8% of WBC -
Treatment (see algorithm below)
Classical HL
Limited stage – favourable:
Plan for ABVD x2 and IFRT 20Gy [Engert, Andre].- If patient is 70 – interim PET (iPET) stratified approach (iPET ~10-14 days after cycle 2B of ABVD)
- If iPET negative (Deauville (D1-3)): continue with plan for IFRT 20Gy. Final restaging with CECT after radiation.
- If iPET positive (D4-5): escBEACOPP x2 additional cycles and IFRT 30Gy. Final restaging with PET/CT after radiation.
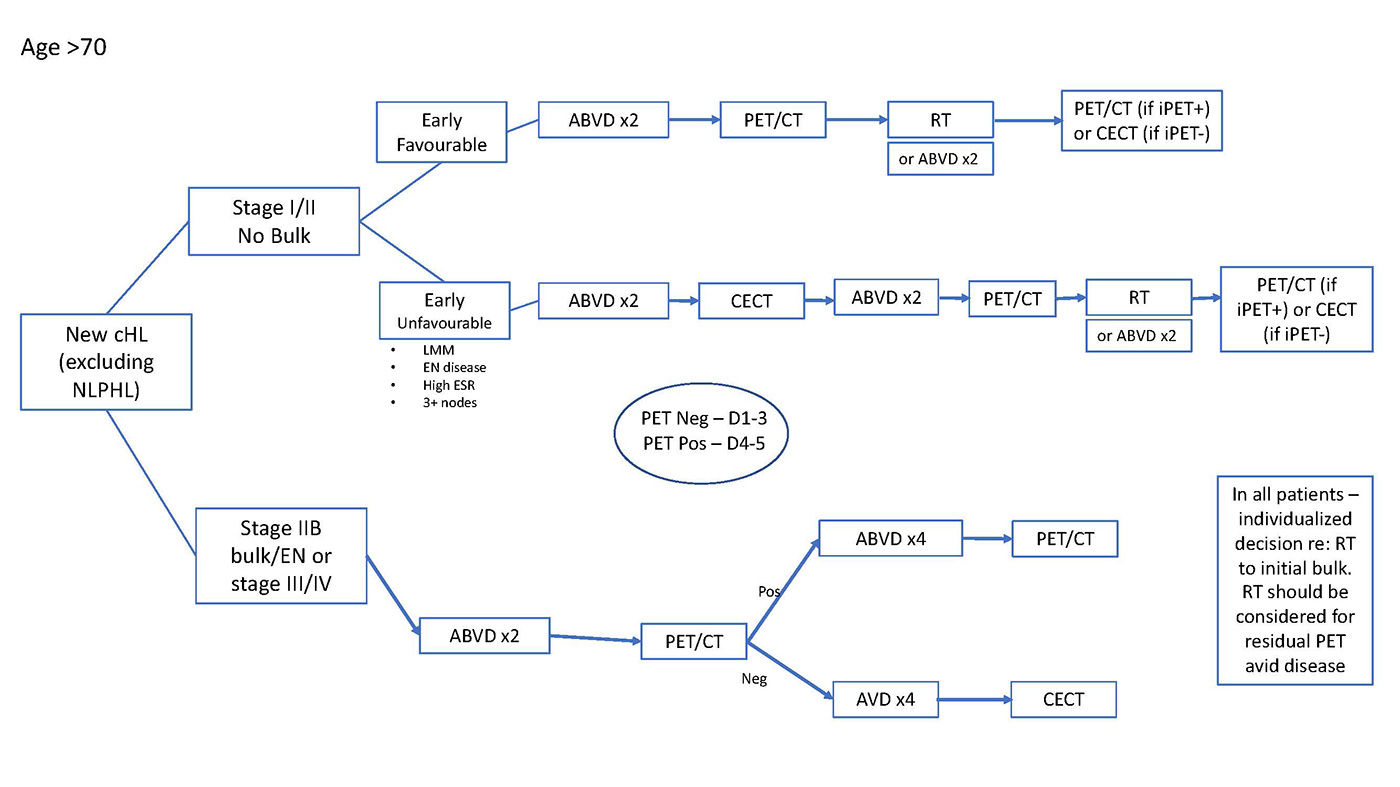
- If patient is >70 – than default regimen is ABVDx2 and IFRT 20Gy. PET/CT prior to radiation may guide radiation dose and extent. Final restaging with PET/CT (if interim PET/CT positive) or CECT (if interim PET/CT negative).
- Individualized risk assessment/discussion around radiation – some patients may be considered for chemotherapy alone (total of ABVD x4 cycles) [Andre, Radford, Meyer]
Plan for ABVD x4 and IFRT 30Gy. [Eich, Andre]
- If patient is 70 – interim PET (iPET) stratified approach (iPET ~10-14 days after cycle 2B ABVD)
- If iPET negative (D1-3): continue with plan to complete ABVD and IFRT 30Gy. Final restaging with CECT after radiation.
- If iPET positive (D4-5): escBEACOPP x2 and IFRT 30Gy. Final restaging with PET/CT after radiation.
- If patient is >70 – than default regimen is ABVDx4 and IFRT 30Gy. CECT after cycle 2 (to ensure there is no evidence of early progression) and PET/CT after cycle 4 may guide radiation dose and extent. Final restaging with PET/CT (if interim PET/CT positive) or CECT (if interim PET/CT negative).
- Individualized risk assessment/discussion around radiation – some patients may be considered for chemotherapy alone (total ABVD x6 cycles) [Andre, Meyer]
- Plan for ABVD and iPET stratified approach. [Johnson]
- ABVD x2 (iPET ~10-14 days after cycle 2B of ABVD)
- If iPET negative (all ages): complete additional AVD x4. Final restaging with CECT.
- If iPET positive and patient 70: escBEACOPP x4 (and RT to residual PET avid areas).
- If iPET positive and patient >70: continue ABVD x4 (and RT to residual PET avid areas).
- Radiation to initial sites of bulk should be individualized. An early referral to radiation oncology is warranted.
- Note – for a subset of individuals who are 60 and with high-risk Hasenclever, a reasonable alternative option is to initiate therapy with escBEACOPP x 2 cycles and assess iPET (day 17-21 of cycle 2):
- Patients with iPET negative continue treatment with escBEACOPP x2 or de-escalate with ABVD x4. (Borchmann, Casasnovas) Patients with iPET positive continue treatment with escBEACOPP x4 cycles (and RT to residual PET avid areas).
- Note – Brentuximab vedotin with AVD therapy (BV-AVD) has been associated with an improvement in modified progression-free survival in patients with advanced stage HL (compared to ABVD). This may be a funded option for patients in late 2021. [Connors]
Supportive/Ancillary treatment:- Allopurinol 300 mg daily x 7 days starting D-1 for cycle 1 only
- ABVD: Filgastim/PegFilgastim primary prophylaxis will not routinely be used with ABVD due to high frequency of asymptomatic neutropenia and low rates of febrile neutropenia. Treatment will not typically be delayed for neutropenia.
- escBEACOPP: Filgastim/PegFilgastim primary prophylaxis will be routinely given.
- Febrile neutropenia should be managed following Febrile neutropenia guideline
- Fertility preservation should be offered to all patients if age appropriate
- Transfusion Considerations: Patients with HL require irradiated blood products
- iPET after x2 cycles of ABVD (ideally x10-14 days after cycle 2B)
- if iPET is negative, can complete final response assessment with CECT
- if iPET is positive, complete final response assessment with PET/CT (ideally ~10-12 weeks post RT)
- Follow visits every 3 months x 2 years, every 6 months x 3 years. Follow up will be transferred back to primary care physician at between years 3 and 5
- At each visit: history and physical exam, CBC, LDH, TSH every 6 months if thyroid irradiation
- Counselling on physical and psychosocial issues, smoking cessation, age-appropriate cancer screening, immunizations
- Screening mammography and MRI starting at years 8 for women who received radiation to the mediastinum/infraclavicular/axillary nodes
- If eligible for high-dose therapy: GDP and referral for autologous stem cell transplant
- Consider IFRT 20-30Gy to sites of bulk at relapse
- If high-risk relapse (refractory disease, early relapse <12 months, relapse with EN disease >=12 months), then consolidate post-ASCT with brentuximab vedotin (BV) (q3 weeks up to 16 cycles)
- IFRT if localized relapse in previously non-irradiated site
- Some patients with a durable response to ASCT and a suitable donor may be considered for allogeneic stem cell transplantation.
- BV for patients with relapse/refractory disease post ASCT
- PD-1 inhibitor therapy for relapse/refractory disease post BV
- IFRT to localized/non-bulky stage 1-2A disease
- Patients with stage IB or 2B and those with advanced stage disease can be treated according to the protocols for classic HL [Savage]
- Patients with NLPHL were generally excluded in studies based on PET stratification; in advanced stage NLPHL, it is reasonable to consider iPET with the view to de-escalate therapy (from ABVD to AVD). The use of iPET to escalate therapy to escBEACOPP is not recommended.
- Consider the potential for transformation to high-grade B-cell lymphoma in patients with rapidly progressive disease, marked B-symptoms, focal splenic lesion, or extranodal sites of involvement.
- If patient is 70 – interim PET (iPET) stratified approach (iPET ~10-14 days after cycle 2B of ABVD)
Useful resources
- IPS risk score (QxMD)
- Patient regimen information
Key references:
- Andre MPE, Grinsky T, Federico M, et al Early positron emission tomography-adapted treatment in stage I and II Hodgkin lymphoma: Final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 2017; 35(16):1786-94.
- Borchmann P, Goergen H, Kobe C, et al.PET-guided treatment in patients with advanced-stage Hodgkin's lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet. 2018; 390(10114):2790-2802.
- Casasnovas RO, Bouabdallah R, Brice P, et al. PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): a randomised, multicentre, non-inferiority, phase 3 study. Lancet Oncol 2019;20(2):202-215.
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32(27):3059-68.
- Connors JM, Jurczak W, Straus DJ et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N Engl J Med 2018; 378:331-344
- Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin's lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28(27):4199-4206.
- Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med. 2010;363(7):640-652.
- Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339(21):1506-1514.
- Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin's lymphoma. N Engl J Med. 2016;374(25):2419-29.
- Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin's lymphoma. N Engl J Med. 2012;366(5):399-408
- Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med 2015;372:1598-60
- Savage KJ, Skinnider B, Al-Mansour M, et al. Treating limited-stage nodular lymphocyte predominant Hodgkin lymphoma similarly to classical Hodgkin lymphoma with ABVD may improve outcome. Blood. 2011;118(17):4585-90
- Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: Field and dose guidelines from the International lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys 2014, 89: 854-862.
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pilari SA, Stein H, Thiele J, Vardimen JW. WHO classification of tumours of haematopoietic and lymphoid tissues. In: World Health Organization Classification of Tumours. Lyon, France: IARC Press, 2016






