Plasma cell neoplasms
Lead: Neil Berinstein
Date of last revision: June 2, 2020
Plasma cell Myeloma:
-
Epidemiology:
There are expected to be over 3000 new cases of multiple myeloma (MM) annually in Canada. MM accounts for about 1 per cent of all cancers and about 10 per cent of hematologic malignancies. Multiple myeloma is slightly more common in men than in women and four times more common in African-Americans. The median age of diagnosis is 65. Almost all patients with MM will evolve from an asymptomatic MGUS. -
Diagnosis:
The diagnosis of MM requires that there are least 10 per cent clonal plasma cells in the bone marrow or an extramedullary plasmacytoma1. In addition, at least one of the following criteria must be met:- Evidence of end organ damage that can be attributed to the underlying plasma cell proliferative disorder, specifically:
- Elevated serum calcium (>2.75 mmol/liter)
- Serum creatinine 177 mmol/L or measured creatinine clearance <40 mL/min
- Anemia: hemoglobin value of >20 g/L below the lower limit of normal, or a hemoglobin value <100 g/L
- Bone lesions: one or more osteolytic lesions (≥5 mm) on skeletal radiography, computed tomography (CT), or positron emission tomography-CT (PET-CT)
- Clonal bone marrow plasma cell percentage ³60 per cent
- Involved/uninvolved serum free light chain (FLC) ratio 100 (involved free light chain level must be 100 mg/L)
- >1 focal lesion on MRI > 0.5 cm
- Evidence of end organ damage that can be attributed to the underlying plasma cell proliferative disorder, specifically:
-
Baseline testing:
When MM is suspected, patients should be evaluated for the presence of M proteins with SPEP, Immunofixation and serum free light chains. About 2% of MM is non-secretory and will be negative on all the above tests. Initial investigations are shown below and include; hematology and biochemistry and 24-hour urine for creatinine, albumin and immune-electrophoresis which will quantitate and characterize renal dysfunction. The extent of bone disease is assessed by low dose whole-body CTT, PET/CT imaging or MRI which is particularly useful for spinal disease. Conventional skeletal survey is less sensitive than low dose WB CTT or PET.- Full history and physical
- CBC, Albumin, LFTs (Bilirubin, ALT, ALP), creatinine
- Hepatitis B surface antigen, Hepatitis B core antibody, Hepatitis C antibody and HIV serology
- Serum protein electrophoresis, quantitative immunoglobulin profile, immunofixation, serum free light chain assay
- Beta-2-microglobulin, uric acid
- 24 hour urine for protein and immunoelectrophoresis
- Tb skin test
- Bone marrow aspiration and biopsy including FISH
- Myeloma survey
- MRI spine and pelvis
- Pro-BNP and cardiac echo if AL amyloid suspected
- strongyloides serology (and stool for ova+parasite) is recommended in patients with myeloma who were born, resided, or traveled long-term (i.e. 6 months cumulative exposure to rural or beach areas, or contact with skin with sand/soil) in the following regions-southeast Asia, Oceania, Sub-saharan Africa, South America, Carribean, Mediterranean countries, Middle East, North Africa, Indian sub-continent, Asia
-
Genetic changes:
There are several recurrent genetic changes that occur in myeloma and may be predictive of high risk behavior and poorer outcome in various trials2,3. These may occur at the time of development of the MGUS clone. The rate of progression of the MGUS clone is influenced by the underlying cytogenetic change and MGUS’s with t(4;14), del 17p and gain 1q are at a higher risk of progression from MGUS or SM to MM. Most of these translocations involve translocation to the immunoglobulin heavy chain on chromosome 14 or the presence of trisomies. These include: t(11;14), t(4:14), t(14;16). In addition, these include deletions of 17p and gains of 1 q or del 1p. These can be detected by fluorescent in situ hybridization (FISH). -
Staging and prognosis (revised International staging system for myeloma):
| Prognostic factor | Criteria | Median Survival |
|---|---|---|
|
ISS Stage |
b-2-Microglobulin <3.5 mg/L & serum albumin ³35 mg/L |
|
| A new Model for risk stratification | ||
|
Chromosomal abnormalities |
FISH |
LDH |
|
R-ISS stage |
ISS stage I and standard risk cytogenetics and normal LDH Not R-ISS stage I or III ISS stage III and either high-risk cytogenetics or high LDH |
83 months (70m-NT, 88m-T) |
Stage is an important predictor of prognosis and this new staging system includes the contribution of high-risk genetic alterations. Median survival is shown above (NT-Non Transplant, T- Transplant). The 5-year overall survival is: 82 per cent for R-ISS stage 1, 62 per cent in R-ISS 2 and 40 per cent in R-ISS 31. Other high-risk features include features of plasma cell leukemia (>2000 plasma cells/microL of peripheral blood or >/= 20 per cent on a manual count.)
| Response | iMWG criteria |
| sCR | CR as defined below plus normal FLC ratio and absence of clonal cells in bone marrow by immunohistochemistry or immunofluorescence |
| CR | Negative immunofixation on the serum and urine and disappearance of any soft tissue plasmacytomas and <5% plasma cells in the bone marrow |
| VGPR | Serum and urine M-protein detectable by immunofixation but not on electrophoresis or >90 per cent reduction in serum M protein plus urine M protein level of <100 mg/24h |
| PR | >50 per cent reduction of serum M-protein and reduction in 24 h urinary M-protein by >90 per cent or to <200 mg/24h If the serum and urine M protein are unmeasurable, a 50 per cent decrease in the difference between involved and uninvolved FLC levels is required in place of the M-protein criteria If the serum and urine M-protein are not measurable, and serum free light chain assay is also not measurable, 50% reduction in plasma cells is required in place of M-protein, provided baseline bone marrow plasma cell percentage was >30 per cent In addition to the above listed criteria, if present at baseline, a >50 per cent reduction in the size of soft tissue plasmacytomas is also required |
| MR | NA |
| Progression | Any one or more of the following criteria: Increase of 25 per cent from lowest confirmed response value in one or more of the following criteria: Serum M-protein (absolute increase must be ≥ 5 g/L); Serum M-protein increase ≥1 g/dL, if the lowest M component was ≥50 g/L; Urine M-protein (absolute increase must be ≥200 mg/24 h); In patients without measurable serum and urine M-protein levels, the difference between involved and uninvolved FLC levels (absolute increase must be >100 mg/L); In patients without measurable serum and urine M-protein levels and without measurable involved FLC levels, bone marrow plasma-cell percentage irrespective of baseline status (absolute increase must be ≥10 per cent); Appearance of a new lesion(s), ≥50 per cent increase from nadir in SPD of >1 lesion, or ≥50 per cent increase in the longest diameter of a previous lesion >1 cm in short axis; ≥50 per cent increase in circulating plasma cells (minimum of 200 cells per μL) if this is the only measure of disease |
Treatment: Figure demonstrating principals of Management of Transplant Eligible and Transplant Ineligible patients with multiple myeloma (credit - EM Berinstein)
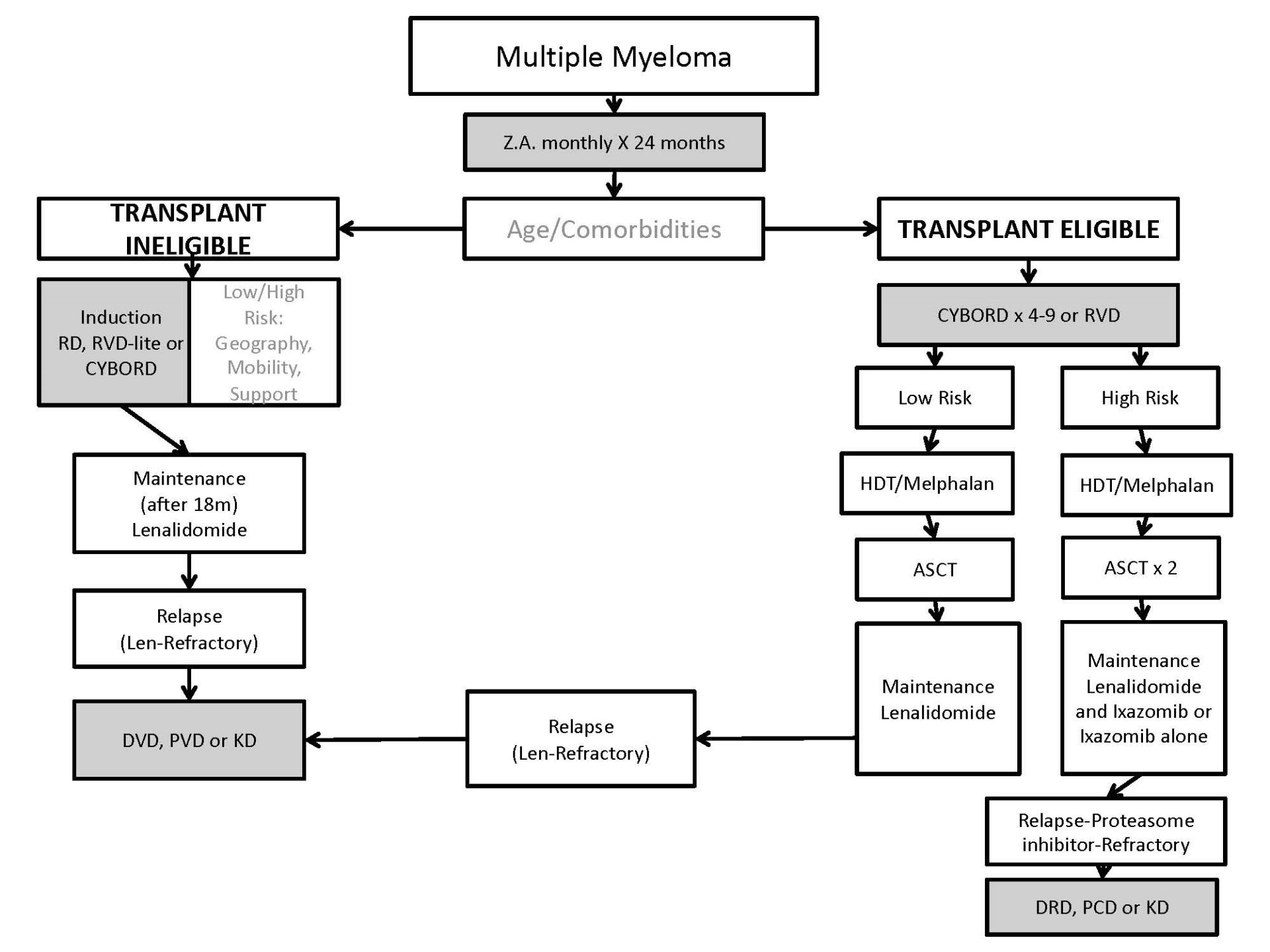
Multiple myeloma -First-line:
Patients with a new diagnosis of MM are categorized as either transplant eligible or transplant non-eligible based upon their age and comorbid illness profile. Toxicities increase the risk of stem cell transplantation patients over than age 70 and in many centres, this is the cutoff age. Patients with major comorbid illness may not be transplant eligible even if under age 70.
-
Transplant Eligible Multiple Myeloma:
Patients eligible for HDT and SCT are treated initially with combination chemotherapy for debulking. At present the current local standard of care in Ontario is CYBORD (cyclophosphamide, bortezomib and dexamethasone)6. Patients usually receive 3-4 cycles (funded in Ontario although can receive more) of this chemotherapy prior to transplant, have their stem cells collected and proceed to high dose melphalan transplantation either as an inpatient or outpatient. The response rate to CYBORD is 95 per cent and the most common grade 3 or greater toxicities were neutropenia at 4 per cent and infection at 10 per cent.
Prior to HDT/SCT, patients must have careful assessment of organ function including pulmonary and cardiac assessments, venous access and dental assessments.
Patients receiving CYBORD receive anti-viral and anti-PJP prophylaxis because of the increased risk of viral infections while on this chemotherapy combination.
Patients receiving high doses of corticosteroids for prolonged periods of time (>/= 20 mg Dexamethasone weekly for greater than 4 weeks in line with NCCN and ECIL guidelines) should also receive prophylaxis against pneumocystis.
Other chemotherapy combinations are acceptable and used in different geographic locations and include RVD -also referred to as VRD (lenalidomide, bortezomib and dexamethasone)7. Chemotherapy combinations with the anti-CD38 monoclonal antibody Daratumumab are also showing promising results in the non- transplant eligible populations and include D-VMP and DRD. The added effectiveness of these in the transplant eligible population have not been established. The Mayo clinic retrospectively looked at several different induction regimens used at their institution and did not find any significant difference in PFS and OS after stem cell transplantation8.
Early randomized clinical trials of ASCT versus non ASCT in Europe showed a clear benefit of ASCT in terms of ORR, PFS and OS9. In some studies, ASCT improved OS in multiple myeloma by 12 months. Several subsequent trials however did not show significant improvements in outcomes other than OR. There is some debate about the merit of early transplantation over delayed transplantation and many centres reserve transplantation until the time of relapse. A trial by the IFM randomized patients to receive VRD x 3 followed by ASCT and additional VRD cycles versus VRD x 8 with ASCT reserved for relapse. Both groups received lenalidomide maintenance for one year. PFS and Quality of life was significantly improved with early ASCT but OS was not10. As treatment approaches, available drugs and biologics evolve for the treatment of myeloma, transplantation regimens including maintenance may need optimization and re-evaluation.
At present in Ontario ASCT is offered to fit patients under age 70 as part of initial therapy before relapse. The transplant related mortality is low (less than 1 per cent) and PFS is optimized.
-
Tandem Transplants:
Patients with high risk genetic changes have a poorer prognosis and shorter PFS after stem cell transplant (see review-Rajkumar, S. American J. Hematology, 2018, 93,1091). High-risk genetics include; t(4;14) t (14;16), t(14;20), del 17P and 1q gain and features of plasma cell leukemia. Although not standard of care in many institutions because the results of several trials are mixed, there is evidence that these individuals may benefit from tandem stem cell transplants. In a phase III trial, an improvement in PFS and OS was seen in standard and high-risk genetics patients and R-ISS stage II and III in favour of tandem transplant patients compared to single transplant (Proc of Ash, Blood, 2017, 130: 401). However, a more recent prospective randomized trial conducted in the US by the Bone marrow transplant clinical trials network compared tandem transplant plus maintenance therapy with single transplant, consolidation and maintenance. There were no significant differences in OS in standard or high-risk groups (Proc ASH, Blood, 2016,128).
In Ontario our current practice is to offer patients with high risk genetics tandem transplants.
-
Transplant ineligible
Transplant ineligible patients are generally older than transplant eligible patients and have more comorbid illnesses such that HDT/ASCT would have a higher risk or morbidity and mortality. Different geographic locations have different first-line standard of care chemotherapies. For the most part, these are IMiD-based. For patients where weekly visits to the cancer centre for subcutaneous bortezomib infusions would be difficult RD (lenalidomide and dexamethasone) is the standard. Although the starting dose of Lenalidomide is 25 mg and of Dexamethasone is 40 m weekly this is often reduced initially until it is clear that it is well tolerated. Patients over age 60 are usually started on Dexamethasone 20 mg weekly. This is continued until disease progression based on the FIRST trial11. Recent data suggests that lowering the dose of both Lenalidomide and stopping Dexamethasone after 18 months is not associated with adverse outcomes. This is recommended practice to avoid long term complications of steroids and to minimize Lenalidomide-related adverse events (Larocca, A. 2018, Blood, 132 (suppl 1) abstract 305). The expected PFS for transplant ineligible myeloma patients that received Lenalidomide and Dexamethasone is 25-35 months based on various trials. This regimen is generally well tolerated although some patients may experience fatigue, gastrointestinal upset and neutropenia. DVT prophylaxis with ASA 81 mg daily is mandatory and there is a small increase in second malignancies. Dexamethasone is associated with insomnia, hyperglycemia and in elderly patients particularly may induce delirium and hallucinations.
More recently RVD-lite (lenalidomide, Dexamethasone and bortezomib-see chemotherapy appendix at end of document) has been made available and is associated with a longer PFS than with Rd alone based on historical comparisons from a single arm Phase 2 trial12. In most patients this is currently the preferred front-line therapy for the transplant ineligible subgroup. However weekly bortezomib subcutaneous injection visits are required and patients may experience neuropathy, increased cytopenias and blepharitis. RVD-lite is continued for 9 months and then patients undergo maintenance therapy with Lenalidomide 10 mg PO daily 21/28 days alone.
There are also promising results with increased PFS with Daratumumab-based front line combinations include D-VMP13 and DRD14 but at present these are not funded in Ontario. Maintenance therapy with Lenalidomide until progression or intolerance is standard. Also 24 months of Zolendronic acid is also standard of care and reduces skeleton-related events (see discussion below).
-
Maintenance therapy:
Maintenance therapy post-transplant has been shown to prolong remissions. From various randomized trials the expected PFS for patients undergoing stem cell transplant followed by maintenance Lenalidomide at 10 mg daily 21/28 reaches 60-70 months. Lenalidomide maintenance is considered standard for patients post ASCT and following induction therapy in a non-transplant setting. A meta-analysis of randomized trials showed a significant improvement in PFS and OS compared to placebo or no therapy (Proc ASCO, J Clin Onc, 2016 supplement, 34, A8001). A concern with Lenalidomide maintenance therapy is a 2-3 x risk of second malignancies (SPMs). Also, patients with high risk genetics may not benefit significantly from lenalidomide maintenance. Maintenance therapy with Lenalidomide 10mg PO daily for 21/28 days indefinitely is recommended post ASCT except for patient with high risk genetics.
For patients with high risk genetics, proteasome inhibitor maintenance therapy may delay progression. The Hovon-4 study showed that Bortezomib subcutaneously every two weeks post-transplant improves OS in standard and high-risk genetics15. Recently the Tourmaline trial showed oral ixazomib maintenance therapy also benefits patients with high risk genetics16. Currently patients in Ontario with high risk genetics are offered maintenance therapy with weekly Ixazomib 3 mg to 4 mg after cycle 4 with or without Lenamlidomide. In current practice, either the lenalidomide or ixazomib is stopped after 1 year so that the patient may have access to a daratumumab triplet using the discontinued drug on progression.
-
Bisphosphonates:
Multiple myeloma is characterized by dysregulated bone metabolism including suppressed osteoblast activity (reduced new bone formation) and enhanced osteoclast (increased bone remodeling and destruction) activity. Bisphosphonates suppress osteoclast activity. Bisphosphonates have been shown to reduce skeletal related events in multiple malignancies associated with bony metastases and in myeloma zolendronic acid may be superior to pamidronate in several outcome measurements including skeletal related events and there is also a small increase in PFS17. In one study, zolendronic acid resulted in a survival advantage over patients treated with clodronate. A meta-analysis did not show a superiority of any single bisphosphonate18. There may be a direct anti-myeloma effect associated with zolendronic acid17. Most recently the RANKL inhibitor Denosumab at 120 mg sc. monthly has been shown to be active and in a post hoc landmark analysis at 15 months showed overall non inferiority to Zolendronic acid for time to skeletal related events19. In this trial, adverse events attributed to the agents included: a doubling of the creatinine from bsaeline-3 per cent denosumab and 7 per cent Zolendonic acid, hypocalcemia-17 per cent denosumab and 12per cent zolendronic acid, and osteornecrosis 1 per cent in both groups. Denosumab is more convenient to administer and can be administered safely in patients with renal impairment but at present is not funded for supportive therapy in multiple myeloma. In addition it is not recommended for patients with creatinine clearances below 30 ml/min. Bisphosphonates and RANKL inhibitors are associated with hypocalcemia, and osteonecrosis of the jaw and poor healing after dental extractions in a low frequency (approximately 1 per cent of patients. We recommend that bisphosphonates should be administered monthly for the first two years of myeloma therapy (as administered in the relevant clinical trials) if they are well tolerated20. There is no clear data on subsequent administration during prolonged remission or relapse and should be stopped at this time. We recommend re-institution of Q3 monthly zolendronic acid at the time of relapse for patients with bony disease. In patients who are commencing completely oral first line therapy (RD) Zolendronic acid can be administered every three months to minimize clinic visits without compromise in efficacy21.
It is recommended that patients undergo a dental assessment before commencing zolendronic acid . If necessary zolendronic acid should be held for three months prior to any elective dental extractions. Renal function must be carefully monitored and doses administered according to creatinine clearance. Denosumab 120 mg sc monthly is an option for patients with impaired renal function (but with Creatinine clearance of greater than 30 mg/ml) or difficult venous access.
-
Summary of OCC recommended first-line therapy: Myeloma
Transplant eligible - CYBORD
- Referral to PMH for auto-transplant opinion
- Zolendronate monthly x 2 years
- Maintenance therapy
Transplant ineligible - RVD-lite until progression (Bortezomib and dexamethasone stopped after 9 months))
- RD may be used in patients felt unable to attend clinic weekly. Dexamethasone is stopped after 18 months and Lenalidomide is reduced to 10 mg 21/28 days.
- Zolendronate monthly x 2 years
- Continuous lenalidomide until progression
-
Recurrent disease
Patients on first line therapy or maintenance therapy are regularly monitored for recurrent/progressive myeloma using serum protein electrophoresis and serum free light chains initially monthly and every three months once there is evidence of disease response and evidence that the drugs are being well tolerated. In patients with secretion of Bence Jones proteins urinary proteins should also be monitored. CBC, LFTS, Calcium and renal function should also be regularly monitored. Bone imaging should be performed to evaluate new persistent musculo-skeletal pains and at least annually. At Sunnybrook, we recommend annual MRI’s of spine and pelvis and annual skeletal surveys.
Asymptomatic biochemical relapse can be managed with adjustments in oral therapy in frailer elderly patients on RD or lenalidomide maintenance. These adjustments could include increasing the dose of lenalidomide, adding weekly Dexamethasone, adding ixazomib or replacing lenalidomide with pomalidomide in combination with dexamethasone or with cyclophosphamide and dexamethasone (PCD). For the most part the results of these adjustments will be transient.
For high risk relapse with CRAB criteria, extramedullary disease, early relapse or other high risk features 22, triplet chemotherapy combinations are preferred over doublets or monotherapy and for the most part have shown improved response durations in randomized trials.
The choice of triplet will depend on the previous treatment details and the patient’s comorbid illnesses. Usually the triplet will be continued until progression or until toxicities require a change.
For patients who have progressed while on a proteasome inhibitor, an IMiD-based salvage combination is preferred. Daratumumab, Lenalidomide and Dexamethasone (DRD) has shown significantly improved response rates and PFS compared to Lenalidomide and Dexamethasone23. All prognostic subsets benefit. KRD could also be used and KRD has superior response rates and PFS to RD24. An alternative combination could be Pomalidomide, Cyclophosphamide and Dexamethasone although this is currently not funded in Ontario25. A limitation of using Carfilzomib or Pomalidomide based triplets as second line is that one would not be able to offer a daratumumab-based combination as third line based on Cancer Care Ontario funding policies (patients would be IMid and proteasome refractory and a triplet would have already been used). Although the use of DRD combination for first relapse will similarly preclude the administration of other triplet combinations (as CCO funding permits only one triplet for relapsed myeloma), carfilzomib could be offered with Dexamethasone as per the Endeavor trial26 and Pomalidomide could also be given with Dexamethasone thus maximizing exposure to salvage regimens with different mechanisms of action. Patients about to start Daratumomab must have samples sent to the transfusion lab for RBC genotyping and a group and screen because circulating Daratumomab may confound blood bank testing.
For patients who have progressed on Lenalidomide, a bortezomib-based combination is preferred. Daratumumab, Velcade and Dexamethasone (DVD) is superior to Velcade and Dexamethasone and has higher response rates and PFS23. Pomalidomide, Velcade and dexamethasone has been tested in a population of patients who are either exposed and/or refractory to Lenalidomide and has shown activity in this population27. In fact, in the lenalidomide refractory subset of relapsing myeloma patients, PVD had a PFS of 9.5 months whereas DVD had a PFS of 7.8 months in a cross-trial comparison suggesting relative comparability of these combinations in lenalidomide refractory subsets. Funded therapeutic options are maximized by using DVD initially for relapse and would allow Pomalidomide to be used in a subsequent line but the reverse is not true. The Carfilzomib and Dexamethasone combination is active in lenalidomide-refractory patients and could be used as second or third line26. DVD would not be available if the lenalidomide refractory patient become refractory to Carfilzomib. The reverse order would be acceptable and KD could be used after progression on DVD. Pomalidomide cyclophosphamide and Dexamethasone could be considered25.
Subsequent lines of therapy would depend on availability of clinical trials and funded new drugs. For example the selective inhibitor of nuclear transport-Selinexor-is available through compassionate use and the the SLAMF7 monoclonal antibody-Elotuzumab- can be accessed through clinical trials.
-
Supportive prophylactic care and premedication during myeloma therapies:
There are many prophylactic measures that can reduce morbidity for patients with multiple myeloma. Prophylactic bisphosphonates or RANKL inhibitors can reduce skeletal related events (see above). Avoiding contrast material in imaging studies and minimizing NSAID use can preserve renal function. Rapid reductions in serum light chains through chemotherapy or even plasmapheresis can reduce cast nephropathy. Ensuring adequate hydration especially when gastrointestinal side effects are occurring can reduce kidney toxicity. Also, careful fluid management and ongoing assessment of cardiac function is important during carfilzomib treatments.
Toxicities associated with many of the myeloma treatment regimens has resulted in use of various prophylactic supportive medicines. To reduce the development of deep venous thrombosis during treatment with lenalidomide daily low dose (81mg) ASA is recommended28. There is still a significant risk for venous thromboembolism despite ASA prophylaxis with lenalidomide (6 per cent with Lenalidomide alone and higher with lenalidomide combinations) and careful observation of this possibility is needed29. Patients at higher risk for venous thropmboembolism may require more aggressive prophylaxis30. Because of the risk of reactivation of herpes Zoster during proteasome inhibitor therapy antiviral prophylaxis with Acyclovir 400 mg Po daily is recommended. H2 blockers (ie. Fomotidine 40 mg daily) and pneumocystis prophylaxis (trimethoprim sulfa 1 PO three times a week) is recommended for patients receiving high dose steroids31. The leukotriene32 inhibitor Montelukast and an antihistamine is recommended to precede Daratumomab treatment by two days to reduce infusion reaction related symptoms. The gastrointestinal toxicity of Selinexor is reduced by H2 inhibitors and anti-nausea medicines to be commenced prior to starting Selinexor33. The Tracking Early Morbidity a d Mortality in Myeloma (TEAMM) trial showed a reduced frequency of febrile episodes and death with Levofloxacin prophylaxis for 36 weeks at the start of myeloma treatment34.
Other plasma cell disorders-Monoclonal gammopathy of Uncertain Significance (MGUS), Smoldering Myeloma (SM) and solitary plasmacytoma:
Almost all patients with Multiple Myeloma progress from asymptomatic phases including MGUS and smoldering myeloma (for review see Go, R. Blood, Jan 11 2018, vol 131, number 2, page 163). MGUS is common with 3 per cent of the population over age 50 meeting the criteria for diagnosis. MGUS progresses to multiple myeloma at a rate of 0.1 per cent to 1 per cent per year depending on the risk profile. SM progresses at a rate of 10 per cent per year of the first five years but then the risk decreases to 3 per cent/year for the next five years and 1.5 per cent/year thereafter. Genetic changes can be found in MGUS or SM and the rate of progression is higher in patients with t(4:14), del 17p or 1q gain. MGUS can be subdivided into IgM MGUS, non-IgM MGUS and light chain MGUS.
-
Diagnostic criteria:
The diagnostic criteria for MGUS, SM and plasmacytoma are shown in the table below and the rate of malignant progression:Entity Diagnostic critieria Risk of progression Pattern of progression IgM MGUS All 3 criteria must be met:
- IgM<30 gr/L
- BM<10% lymphoplasmacytic infiltration
- No evidence anemia, B sx, hyperviscosity, adenopathy, or hepatosplenomegaly
1 per cent/year Waldenstrom’s macroglobulinemia AL amyloidosis, rarely MM Non IgM MGUS All 3 criteria must be met:
- Serum monoclonal Non IgM Ig <30 g/L
- Clonal PC < 10 per cent
- No CRAB criteria
0.5 per cent /year Multiple myeloma, solitary plasmacytoma or AL amyloidosis Light Chain MGUS All criteria:
- Abn FLC ration <0.26 or >1.65
- Increased level of involved light chain
- No heavy chain abn protein on IFA
- No CRAB
- Clonal PC less than 10 per cent
- Urinary monoclonal protein <500 mg/24
0.3 per cent/year Light chain multiple myeloma or AL amyloidosis SM Both criteria must be met
- Serum IgG or IgA >/= 30 grams/L or urinary protein >500 mg/24 hour or clonal PC between 10-60 per cent
- No CRAB or amyloid related findings
10 per cent/year x 5 years, 3 per cent/year x 5 years then 1.5 per cent/year x 5 years Multiple myeloma or AL amyloid Plasmacytoma All criteria must be met:
- Bx proven bone or soft tissue lesion with clonal plasma cells
- Normal bone marrow
- Normal skeletal survey, MRI (or CTT) of spine and pelvis except for primary lesion
- No CRAB
30-50 per cent over 10 years Multiple myeloma Plasmacytomas can be solitary boney plasmacytomas (SBP) or extramedullary plasmacytomas (EMP)35. 50 per cent of SBP and 30 per cent of EMP develop MM within 10 years. 50 per cent of patients with plasmacytomas will have a small M protein (<5 g/L) and 40 per cent will have an abnormal SFLC ratio.
-
Baseline work-up:
- CBC, renal function, Calcium, albumin
- Serum protein electrophoresis
- Immunofixation
- Serum k and l light chains and ratio
- 24 hour Urine protein electrophoresis and immunoelectrophoresis for protein quantitation
- Spot urine for detection of Bence Jones proteins
Patients are classified as low or high risk based upon the initial testing36.
Low Risk MGUS High Risk MGUS Type of paraprotein IgG, l, or k IgM, IgA Level of paraprotein <15 grams/L >10 grams/- IgM, >15 grams- IgG, IgA Serum free light chain ratio37 < 8 for k or > 8 for l >8 for k or < 8 for l Immunoparesis38 Normal uninvolved Ig Low uninvolved Ig Risk of progression 0.1-0.5 per cent–(2 per cent life-long risk) >1 per cent risk Management Repeat protein studies at 6 month-if stable, to be followed by family MD Perform BM asp and biopsy, skeletal survey and MRI spine and pelvis. Follow SPE and SFLCs annually and repeat imaging with repeated progressive rises
Low risk MGUS patients have a 0.1-0.5 per cent risk of developing myeloma with a life time risk of 2 per cent and do not require further investigations assuming they are asymptomatic (deny new boney pains). The likelihood of finding PC in the bone marrow of these patients was very low (<4.7 per cent) and the likelihood of finding skeletal lesions was <2.5 per cent They can be re-assessed in 6 months and if stable do not need further follow-up by a hematologist unless they develop symptoms of concern. Low risk patients over age 80 whose paraprotein or light chains remain stable for 6 months have a higher risk of mortality from some other cause and also do not need further follow-up by a hematologist. High risk MGUS patients have a greater than 1 per cent/year chance of malignant progression to SM, MM or WM and should have further initial work-up including a bone marrow aspiration and biopsy, an MRI of spine and pelvis and myeloma survey. Patients with an IgM paraprotein do not need skeletal imaging. High risk patients should be followed annually with CBC/creatinine, SPE, serum free light chains and ratio and urinary protein. Imaging or bone marrow assessments should only be performed if the patient develops symptoms of concern, or a progressive rise in paraprotein or light chains over three consecutive measurements.Patients with plasmacytomas should have SPEs, SFLCs and ratios, bone marrow aspirations and biopsies including flow cytometry for clonally restricted plasma cells, myeloma survey and MRI of the spine and pelvis. Abnormalities of any of these tests could suggest systemic disease with multiple myeloma.
-
Treatment:
Treatment is not offered to patients with low or high risk MGUS or smoldering myeloma (although clinical trials to explore the merit of various treatments in SM are underway). If myeloma develops the appropriate treatment of myeloma is initiated. SBPs and EMPs are very radiosenstive and are treated with radiotherapy although surgery may be used initially for diagnostic purposes or for stabilization of the spine or other bones. Doses of radiation equal or greater than 40 Gy are usually used and are associated with low local recurrence rates. Persistent elevations or recurrences of the M protein or SFLCs post radiation therapy predicts for development of multiple myeloma that will require systemic therapy. Patients should be followed at 6 months with imaging of radiated plasmacytoma and then annually with imaging of plasmacytoma, MRI of spine and pelvis and with skeletal survey.
Other plasma cell disorders-AL Amyloid:
There are a number of different types of amyloid all of which are cause by the mis-folding and aggregation of protein fibrils in various tissues. These may be hereditary or acquired and involve the misfolding of various proteins into b-pleated sheet structures. Although AL amyloid is the most common (approximately 70 per cent) and is associated with plasma cell neoplasms, it must be differentiated from two other relatively common type of amyloids including ATRA amyloid caused by mutations in the transthyretin protein which can by acquired or inherited) and AA amyloid caused by relatively high levels of serum amyloid protein associated with chronic inflammatory disorders.
In the USA the incidence of AL Amyloid is low at 8.9 per 106 people. The mean age of diagnosis for AL amyloid is 63. The major risk factor for AL amyloid is MGUS, smoldering myeloma and multiple myeloma. Approximately 1 per cent of patients diagnosed with multiple myeloma will eventually develop AL amyloid. For patients with MGUS the relative risk of developing amyloid is 8.8 compared to those without MGUS.
There are four criteria considered essential and required by the International Myeloma Working Group (IMWG) to diagnose AL amyloid39:
- Presence of an amyloid -related systemic syndrome symptomatology including most significantly CHF, nephrotic syndrome, polyneuropathy, and/or organomegaly
- Positive amyloid staining by congo red in any tissue
- Evidence that amyloid is light chain related by direct examination of the amyloid using mass spectography-based proteomic analysis or IEM
- Evidence of a monoclonal plasma cell proliferative disorder (serum or urine monoclonal protein, abnormal FLC ratio or clonal plasma cells in the bone marrow)
AL amyloid may be localized or systemic. Localized amyloid is felt to be caused by local plasma cell production of the AL protein. It is very indolent not usually requiring systemic therapy although there may be long term damage to the involved organ. The most common sites of localized AL amyloid include skin, larynx, lung, bowel, orbit and urinary tract. Localized amyloid does not usually progress into systemic amyloid.
AL amyloid is often associated with chromosomal alterations. Of the chromosomal alterations, t(11:14) occurs in 50 per cent, monosomy/del 13q in 36 per cent and trisomies in 26 per cent. t(11:14) is associated with a poor prognosis40. t(4:14) , t(14:16) and P53 abnormalities are not associated with a poor prognosis in AL amyloid.
Several different risk stratification indices have been proposed. Cardiac involvement is the most significant prognostic factor. Patients undergoing HDT/ASCT with an elevated troponin had a mortality rate-as high as 28 per cent and criteria using troponin have been used to exclude patients from ASCT41. Using the revised Mayo clinic staging criteria which incorporates dFLC’s, CTnT, and NT-proBNP four stages were created with OS from 94 months to 5.8 months42. Stage was based on the number of risk factors (0-3).
Treatment for systemic amyloid needs to be multi-disciplinary and include supportive management by cardiologists, nephrologists and neurologists for dyspnea, edema and neuropathy.
In low risk patients (stage one or single organ) who have a good hematologic response to systemic therapy HDT and ASCT may induce deep responses that may reverse or reduce ongoing organ damage from the amyloid protein (for review see43). In one of the larger ASCT trials involving 89 patients, patients were randomized to melphalan and dexamethasone or HDT/SCT. There was a bias in selection of patients for ASCT. The 3 year PFS for the melphalan and dexamethasone was 29 per cent and OS 58.8 per cent while the HDT/SCT arm had a 3 yr PFS of 51.7 per cent and OS of 83.6 per cent.
Systemic therapy is aimed at quickly reducing the amyloid cell clone and amyloid protein. Currently the most accepted therapy is proteasome inhibitor based and CYBORD is often used. The response rate to CYBORD 60-94 per cent in two different trials. CYBORD may be used as induction pre-transplant and for non-transplant eligible patients44. Other proteasome inhibitors and imods also are active and can be used at relapse. Recently Daratumumab has shown activity in AL Amyloid45.
| Summary of OCC-recommended first line therapy Amyloidosis | ||
|---|---|---|
| First line therapy: CYBORD to be followed by ASCT in transplant eligible patients without multisystem dysfunction from Amyloid | ||
| Maintenance: therapy with Bortezomib every second week or weekly ixazomb until progression or toxicity |
Other plasma cell disorders-plasma cell leukemia:
Although very rare (0.04 cases per 100,000) plasma cell leukemia (PCL) is the most aggressive subtype of plasma cell neoplasms. The diagnosis is based on an absolute plasma cell count in the peripheral blood of 2 x 109/L and more than 20 per cent circulating clonal plasma cells. Lower levels of plasma cells may define a risk factor in multiple myeloma. PCL may be either primary or secondary where it is seen in relapsed or refractory MM. PCL has distinct clinical features from MM with cytopenias and infiltration of extramedullary sites being common and osteolytic bone involvement being less common. Renal insufficiency, high LDH and Beta 2 microglobulin and hypercalcemia are also seen. Historically PCL was associated with a median OS of less than 1 year but this has improved with stem cell transplant and newer agents46.
-
Treatment of Plasma cell leukemia:
Patients who are transplant eligible should be treated with a regimen than combines cytotoxic drugs with myeloma-specific drugs. One such regimen is VTD or VRD-PACE (bortezomib, thalidomide or lenalidomide and Dexamethasone with cisplatinin, doxorubicin, cyclophosphamide and etoposide)47. This should be followed by ASCT. For patients who are not transplant eligible a bortezomib-Imid based such as RVD or RVD-lite for frailer patients is recommended48. In all cases adequate hydration and tumour lysis prophylaxis with allopurinol or rasburicase are also recommended.
Secondary PCL is associated with drug resistant refractory MM and has a poor OS of usually approximately 1 month and unless trials of novel agents are available a palliative approach is recommended.
Summary of OCC-recommended first line therapy for plasma cell leukemia: Transplant eligible: VRD -PACE followed by transplant followed by Lenalidomide maintenance Transplant ineligible: RVD or RVD-lite followed by maintenance with bortezomib every 2 weeks
References:
- Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863-2869.
- Kumar SK, Rajkumar SV. The multiple myelomas - current concepts in cytogenetic classification and therapy. Nat Rev Clin Oncol. 2018;15(7):409-421.
- Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(5):548-567.
- Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467-1473.
- Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328-e346.
- Areethamsirikul N, Masih-Khan E, Chu CM, et al. CyBorD induction therapy in clinical practice. Bone Marrow Transplant. 2015;50(3):375-379.
- Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527.
- Paquin AR, Kumar SK, Buadi FK, et al. Overall survival of transplant eligible patients with newly diagnosed multiple myeloma: comparative effectiveness analysis of modern induction regimens on outcome. Blood Cancer J. 2018;8(12):125.
- Ntanasis-Stathopoulos I, Gavriatopoulou M, Kastritis E, Terpos E, Dimopoulos MA. Multiple myeloma: Role of autologous transplantation. Cancer Treat Rev. 2020;82:101929.
- Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017;376(14):1311-1320.
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906-917.
- O'Donnell EK, Laubach JP, Yee AJ, et al. A phase 2 study of modified lenalidomide, bortezomib and dexamethasone in transplant-ineligible multiple myeloma. Br J Haematol. 2018;182(2):222-230.
- Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med. 2018;378(6):518-528.
- Facon T, Kumar S, Plesner T, et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med. 2019;380(22):2104-2115.
- Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012;30(24):2946-2955.
- Dimopoulos MA, Gay F, Schjesvold F, et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2019;393(10168):253-264.
- Morgan GJ, Davies FE, Gregory WM, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376(9757):1989-1999.
- Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012(5):CD003188.
- Raje N, Terpos E, Willenbacher W, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19(3):370-381.
- Terpos E, Roodman GD, Dimopoulos MA. Optimal use of bisphosphonates in patients with multiple myeloma. Blood. 2013;121(17):3325-3328.
- Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients With Bone Metastases: A Randomized Clinical Trial. JAMA. 2017;317(1):48-58.
- Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149-157.
- Dimopoulos MA, San-Miguel J, Belch A, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX. Haematologica. 2018.
- Dimopoulos M, Wang M, Maisnar V, et al. Response and progression-free survival according to planned treatment duration in patients with relapsed multiple myeloma treated with carfilzomib, lenalidomide, and dexamethasone (KRd) versus lenalidomide and dexamethasone (Rd) in the phase III ASPIRE study. J Hematol Oncol. 2018;11(1):49.
- Baz RC, Martin TG, 3rd, Lin HY, et al. Randomized multicenter phase 2 study of pomalidomide, cyclophosphamide, and dexamethasone in relapsed refractory myeloma. Blood. 2016;127(21):2561-2568.
- Ludwig H, Dimopoulos MA, Moreau P, et al. Carfilzomib and dexamethasone vs bortezomib and dexamethasone in patients with relapsed multiple myeloma: results of the phase 3 study ENDEAVOR (NCT01568866) according to age subgroup. Leuk Lymphoma. 2017;58(10):2501-2504.
- Richardson PG, Oriol A, Beksac M, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(6):781-794.
- Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22(2):414-423.
- Chakraborty R, Bin Riaz I, Malik SU, et al. Venous thromboembolism risk with contemporary lenalidomide-based regimens despite thromboprophylaxis in multiple myeloma: A systematic review and meta-analysis. Cancer. 2020;126(8):1640-1650.
- Li W, Garcia D, Cornell RF, et al. Cardiovascular and Thrombotic Complications of Novel Multiple Myeloma Therapies: A Review. JAMA Oncol. 2017;3(7):980-988.
- Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J. 2014;44(12b):1350-1363.
- Plesner T, Krejcik J. Daratumumab for the Treatment of Multiple Myeloma. Front Immunol. 2018;9:1228.
- Gavriatopoulou M, Chari A, Chen C, et al. Integrated safety profile of selinexor in multiple myeloma: experience from 437 patients enrolled in clinical trials. Leukemia. 2020.
- Drayson MT, Bowcock S, Planche T, et al. Levofloxacin prophylaxis in patients with newly diagnosed myeloma (TEAMM): a multicentre, double-blind, placebo-controlled, randomised, phase 3 trial. Lancet Oncol. 2019;20(12):1760-1772.
- Fotiou D, Dimopoulos MA, Kastritis E. How We Manage Patients with Plasmacytomas. Curr Hematol Malig Rep. 2018;13(3):227-235.
- Turesson I, Kovalchik SA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood. 2014;123(3):338-345.
- Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106(3):812-817.
- Landgren O, Hofmann JN, McShane CM, et al. Association of Immune Marker Changes With Progression of Monoclonal Gammopathy of Undetermined Significance to Multiple Myeloma. JAMA Oncol. 2019.
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-548.
- Bochtler T, Hegenbart U, Kunz C, et al. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. J Clin Oncol. 2015;33(12):1371-1378.
- Gertz M, Lacy M, Dispenzieri A, et al. Troponin T level as an exclusion criterion for stem cell transplantation in light-chain amyloidosis. Leuk Lymphoma. 2008;49(1):36-41.
- Lakshman A, Rajkumar SV, Buadi FK, et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 2018;8(6):59.
- Vaxman I, Gertz M. Recent Advances in the Diagnosis, Risk Stratification, and Management of Systemic Light-Chain Amyloidosis. Acta Haematol. 2019;141(2):93-106.
- Palladini G, Sachchithanantham S, Milani P, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126(5):612-615.
- Kaufman GP, Schrier SL, Lafayette RA, Arai S, Witteles RM, Liedtke M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood. 2017;130(7):900-902.
- Gonsalves WI, Rajkumar SV, Go RS, et al. Trends in survival of patients with primary plasma cell leukemia: a population-based analysis. Blood. 2014;124(6):907-912.
- Fernandez de Larrea C, Kyle RA, Durie BG, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27(4):780-791.
- Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116(5):679-686.
Acknowledgements: Thanks to Matt Cheung, Irina Amitai, Ivan Tyono, Alia Thawer, Rena Busckstein and Kevin Imrie for their helpful input.
Appendix-Chemo protocols:
- RD
- Lenalidomide 25 mg PO 21/28 days
- Dexamethasone 40 mg PO weekly
- Continue until progression-Dexamethasone may be dropped at 18 months and Lenalidomide dose reduced to 10-15 mg 21/28 days
- Daily ASA 81 mg prophylaxis
- CYBORD
- Bortezomib 1.3 mg/m2 SC D1, 8, 15, 22
- Cyclophosphamide 300 mg/m2 PO D1, 8, 15, 22
- Dexamethasone 40 mg PO daily x 4 D1-4, 9-12, 17-20 for Cycles 1 and 2 Then only daily x 1 D1-4, 9-12, 17-20 Q28 days
- Acylcovir prophylaxis
- ASA 81 mg prophylaxis
- RVD-lite (Sunnybrook)*
- Lenalidomide 15 mg PO daily D1-21/28 days
- Bortezomib 1.5 mg/m2 SC on Days 1, 8, 15
- Dexamethasone 20 mg Po daily x 2 Days 1, 8, 15, 22 (can be daily x 1 for patients older than 75)
- Administered x 9 cycles then Lenalidomide 15 mg PO daily Q21/28
- ASA 81 mg PO daily prophylaxis
- Rabeprazole 20 mg daily
- Valcyclovir 500 mg daily
- Septra DS 1 PO 3x/wk
- After 9 months Bortexomib is discontinued and patients remain on maintenance Lenalidomide
- DVD (Castor)*
- Bortezomib 1.3 mg/m2 SC D1, 4, 8, 11
- Dexamethasone 20 mg PO D1, 2, 4, 5, 8, 9, 11, 12
- Daratumumab 16 mg/kg IV weekly Q21 days x 3 then D1 IV Q21 days x 5 then Q28 days
- Acylcovir prophylaxis
- DRD (Pollux)*
- Lenalidomide 25 mg PO daily D1-21/28 days
- Dexamethasone 40 mg PO D1, D8, D15, D22
- Daratumumab 16 mg/kg IV weekly D1, 8, 5, 22 x 2 cycles then D1, 15 x 4 cycle then Q28 days
- ASA 81 mg PO daily prophylaxis
- PVD (Optimismm)*
- Bortezomib 1.3 mg/m2 SC D1, D4, D8, D11 Q21 x 8 then D1, D8
- Dexamethasone 20 mg PO D!, D2, D4, D5, D8, D9, D11, D12 (10 mg if age >75)
- Pomalidomide 4 mg PO daily D1-14 Q21 Days.
- PCD
- Pomalidomide 4 mg PO daily Q21/28
- Cyclophosphamide 300 mg Po weekly Day 1, D8
- Dexamethasone 40 mg PO Day 1-4, D15-18 x 4 cycles then 40 mg PO Day1, D8, D15, D21
- KD
- Carfilzomib 56 mg/m2 IV D1, D2, D8, D9, D15, D16 (reduced to 20 mg/ m2 on D1, D2 of Cycle 1 only) Q 28 days
- Dexamethasone 20 mg PO D1, D2, D8, D9, D15, D16, D22, D23
*Sunnybrook regimens have been modified






