Features
Messages
Digest
Short Stories
How Fractures Heal
A clinician-scientist explores the biological intricacies of how injured bones get better, and guess what? It’s all about your immune system

Dr. Diane Nam examines John Klotz in the fracture clinic. Nam studies the molecular and cellular aspects of fracture healing at Sunnybrook Research Institute.
Photo: Doug Nicholson
On average, it takes at least six weeks for a fracture to heal in an adult, followed by weeks to months of rehabilitation to regain motion and strength. Moreover, bone injuries that do not heal properly often need surgery to repair the fractured bone.
Dr. Diane Nam, an orthopaedic surgeon and associate scientist in Biological Sciences and the Holland Bone and Joint Research Program at Sunnybrook Research Institute (SRI), and colleagues conducted a preclinical study to investigate the synergy of two systems: the musculoskeletal system and the immune system. The study looked at how various cytokines (cell-signalling protein molecules) expressed by cells in the immune system altered bone healing. It was the first study to show how a specific immune cell could improve osteogenesis, or the formation of bone.
“Our hypothesis was that the immune system played a critical role during the early phase of fracture repair,” says Nam, who is also an assistant professor of surgery at the University of Toronto. “Anytime we have an injury, whether it’s a cut on your skin or a broken bone, the immune system is required.”
Using a mouse fracture model, the researchers examined the rate, quality and strength of fracture healing in immune-deficient mice lacking the T cells and B cells that are critical to a healthy immune response, and compared them with normal mice.
After careful analysis of the biological processes, the study showed that the fractures of immune-compromised mice did not heal as well as those of normal mice. The researchers found that a lack of T cells delayed fracture healing and decreased bone formation.
Nam also looked at the role of inflammatory cytokines in fracture repair. She found that the cytokine IL-17F (produced by T-helper cells) was crucial to bone healing by causing osteoblasts, bone-forming cells, to mature and stimulate bone formation. These findings were published in the journal PLoS ONE in 2012.
“The involvement of IL-17F and osteoblasts in fracture healing is novel, and I think it’s a significant finding. Having new insight into the actual mechanism of fracture healing is a huge step in terms of understanding bone repair pathways,” Nam says. These results may lead to optimizing fracture repair techniques within the next decade and ultimately improve treatments for patients with impaired bone healing, she says.
Although preclinical fracture modelling is taking place around the world, Nam is the first researcher to do this work at SRI. She says it’s tough to find a niche for research in fracture repair and immunology, and is fortunate to have found such a perfect fit at Sunnybrook.
Nam’s research is still in the early stages. The next step will be to outline the exact signalling cascade and assess the functional significance of IL-17F to understand better how it is “doing its magic” in fracture healing, she says.
Fracture repair in action
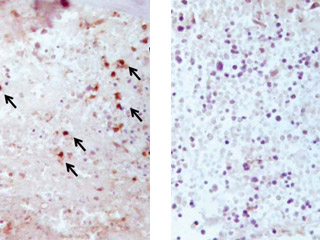
Left: A microscopic image of the bone callus tissue three days after a fracture in normal mice shows abundant staining and presence of T cells (black arrows).
Right: In contrast, in mice lacking T cells, this image shows a complete absence of T cell staining, with no recruitment of T cells in the bone callus tissue three days after a fracture.
Images: submitted by Dr. Diane Nam
Nam also leads an orthopaedic trauma practice at Sunnybrook Health Sciences Centre. She spends at least two days a week treating patients, and specializes in upper extremity reconstruction and limb fractures. Her goal is to help patients heal and function better, earlier, especially those who have impaired healing, and enable them to get back to their daily activities.
“Most people will have a fracture in their lifetime, and when you have something broken or you’ve lost function of an arm or a leg, it really limits your mobility and independence,” she says. “The collective cost to society from lost productivity is huge and one of the largest health care burdens in Canada, not only with the younger trauma population, but also with the growing elderly population.”
Many patients who are elderly have osteoporosis and health issues such as diabetes or cancer, which, in addition to advanced age, contribute to impairment of their immune response. “If you’re one of the patients in the 5% to 10% range that show impaired or delayed healing, then you’re going to spend more time with doctors and hospitals trying to rectify this problem, and it usually involves some type of surgical intervention,” Nam says.
Technology and implants have improved orthopaedic care by helping patients regain some early mobility, but the biological course of fracture healing remains slow. “Surgically we can provide stability internally that allows you to be free from casts, but your ability to weight-bear, to do physical activities like sports, to walk or return to work, is still restricted to a significant degree,” she says.
Well-positioned by her joint role as clinician and researcher, Nam is determined to uncover the biological mechanism of how bones heal, and to apply the findings to clinical care.
“We want to make the problems resulting from fractures go away,” Nam says. “The connection that’s opened up for immunology, bone biology and fracture healing is substantial. In the future, a pharmacological therapy may be possible where you could potentially be able to give someone a drug at the time they break their bone and say, ‘Take these for five days and you’re good to go.’”
— Eleni Kanavas
Nam’s research was funded by the Canadian Institutes of Health Research, a Roscoe Reid Surgical Science Scholarship Award and the University of Toronto.

- << Short Stories |
- Previous: Over the Threshold •
- Next: Multiple Options
- |Connections >>



