Predicting Fracture Risk
Taking the guesswork out of radiotherapy technique

Dr. Arjun Sahgal (left) and Dr. Cari Whyne have partnered to understand why some patients develop a spinal fracture after undergoing stereotactic body radiotherapy and how that risk can be minimized.
Photo: ©Nation Wong
Picture a cancer remedy that targets a tumour with extreme precision and effectively spares surrounding healthy tissues to minimize harmful side effects. Moreover, instead of undergoing months of therapy, imagine completing treatment in two weeks.
Sounds good, doesn’t it?
Stereotactic body radiotherapy (SBRT) is an emerging treatment designed to work this way. Like conventional radiotherapy, SBRT uses high-energy radiation to damage the DNA of cancer cells, thus curtailing the cells’ reproductive spree. However, instead of giving small doses of radiation over many weeks, SBRT delivers high doses of focused radiotherapy in a few days or weeks.
“We’re early adopters [of SBRT],” says Dr. Arjun Sahgal, a scientist in the Odette Cancer Research Program at Sunnybrook Research Institute (SRI) and a radiation oncologist at Sunnybrook. “It’s standard practice for selected indications. Our research focus is to develop new indications and ways to roll it out to more patients because it’s a highly effective therapy and a new paradigm in radiation oncology.”
The technique uses 3-D imaging like computed tomography (CT) and magnetic resonance imaging to pinpoint the target area and plan treatment. The therapy is delivered at several angles and planes, which enables a greater dose to be given. Use of SBRT is normally limited to small, well-defined cancers in the body, including those of the lung, liver, spine, abdomen and prostate.
Sunnybrook has offered stereotactic radiosurgery for brain tumours for many years. In a 2015 study involving patients with brain metastases aged under 50 years, Sahgal showed that the therapy alone extended survival to 13.6 months, versus 8.2 months for patients who had stereotactic radiosurgery plus whole brain radiation treatment.
In 2007, the hospital launched an SBRT program for body sites, initially for spine and lung tumours. The program has since expanded to include treatment of cancers of the kidney, liver and prostate, and cancers that have spread to only a few sites.
In the spine, Sahgal says he is seeing local control rates of about 80% one year after treatment, even in tumours that are highly resistant to radiation, like kidney cancer metastases. A 2012 study by researchers in Japan showed the five-year survival rate of patients with lung cancer who were treated with SBRT was 41%, compared with survival rates of 13% to 22% for patients who had standard radiotherapy.
Although SBRT shows promise, it’s not without risks. In the case of tumours that have spread to the spine, a troubling complication is vertebral compression fracture, which can cause debilitating pain, reduce mobility and necessitate surgery. Sahgal’s research has shown that spinal fractures brought on by SBRT typically occur within three to six months of treatment, and are likely caused by the intensity of the radiation.
Sahgal and Dr. Cari Whyne, director of the Holland Bone and Joint Research Program at SRI, did a literature review published in 2014 in Lancet Oncology that showed the risk of spinal fracture after SBRT ranges from 11% to 39%, worrisome figures given that with conventional radiotherapy the risk of this complication is much lower—about 5%. In light of these findings, they sought to understand who is most likely to have a spinal fracture after SBRT.
A big piece of the puzzle is the amount of tumour in the bone itself, says Whyne, who is also a professor in the department of surgery at the University of Toronto. Her lab has engineered software that calculates the tumour volume in the spine based on a CT scan. “What we have developed over the years is a way to quantify the tumour volume within these vertebrae. When you do a volumetric quantification using this CT data, that can help us identify patients that may indeed go on to fracture versus those that don’t.”
She and Sahgal, who is also a professor at U of T, used the software to analyze the CT scans of patients with tumours that had spread to the spine who were treated with SBRT. They then looked at clinical data on the patients’ outcomes to determine whether there was a threshold beyond which fracture was likely to occur. “It’s really about volume: How much tumour is there? How much bone is missing that’s been replaced by tumour?” says Whyne.
As they discovered, it turns out that the threshold, or amount of disease that can predict fracture risk, is between 10% and 15% tumour volume.
Having a standard measurement takes the guesswork out of identifying which patients may go onto to have a spinal fracture after SBRT—something that has not been done before, notes Sahgal.
For instance, clinicians now use a classification system to determine a patient’s risk for spinal instability. One of the problems with it, however, is the variability in evaluating one of the risk factors—the amount of lytic disease, or the amount of bone that’s been destroyed by a tumour.
“The way we were looking at the spinal instability neoplastic score is if something is 50% lytic versus less than 50% lytic—but there’s no way of knowing what that is. It’s judged by the clinician’s eyeballing of 2-D scan slices. We’re using subjectivity in complex medical decision-making. We have to take the subjectivity out and put in quantifiable metrics,” says Sahgal.
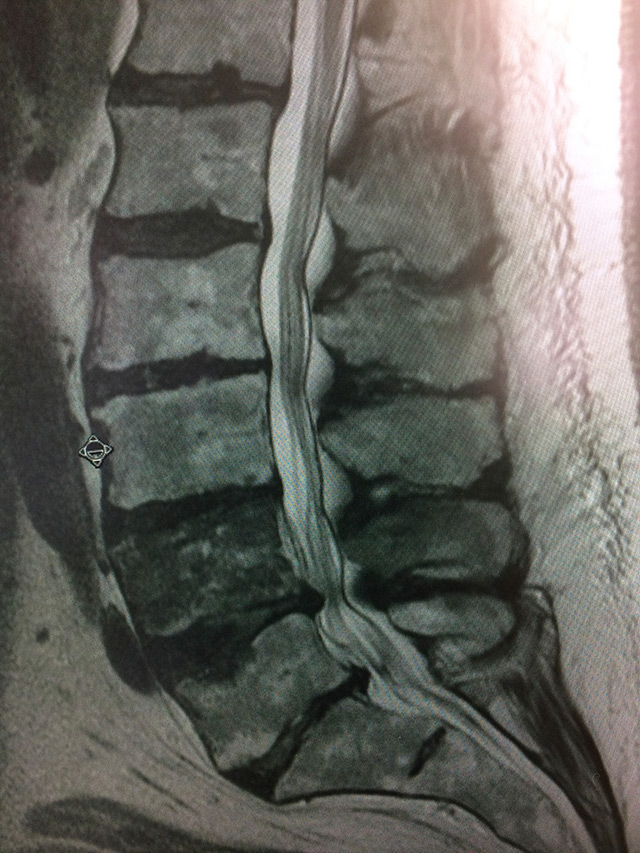
In this spinal column, cancer has spread to the fifth and lowest vertebra of the lumbar spine, called L5. Almost 40% of patients with cancer develop spinal metastases. Stereotactic body radiation therapy shows promise as a treatment.
Photo: ©iStock.com/Spondylolithesis
Whyne says they will also try to uncover why, biologically, spinal fractures occur after SBRT. “We’re trying to understand it from a basic biomechanics perspective—the mechanism for why this is happening. Is there something we can do to reduce the impact or effects?”
Whyne developed the software for research, but she and Sahgal are working with a Toronto-based medical imaging company to develop it into a tool that can help clinicians with treatment planning.
The technology could, for example, inform a radiation oncologist’s decision to recommend a spine stabilization procedure before getting SBRT, says Sahgal.
“Right now, we have no idea what the ideal clinical treatment workflow is for the patient based on their vertebral characteristics.” Moreover, the software may have broader clinical applications, he adds. “Ultimately it goes beyond radiation. It goes to surgeons or any oncologic assessment where you’re looking at the vertebrae and somebody who has pain, and saying, ‘What is the best therapy for this person?’”
In Canada, SBRT is offered in British Columbia, Alberta, Manitoba, Ontario and Quebec, generally only in large cities. Although Sunnybrook is an early provider of the therapy, Whyne says rigorous study is needed to improve patient outcomes. “We’re trying to understand and optimize it so that it benefits those patients who will benefit from it, and so that we change what we do for patients who may have complications.”
Sahgal’s research is supported by the Brain Tumour Foundation of Canada.
Whyne’s research is supported by the AO Foundation, Canadian Breast Cancer Foundation, Canadian Institutes of Health Research, Federal Economic Development Agency for Southern Ontario, Natural Sciences and Engineering Research Council of Canada, and Ontario Centres of Excellence.