Restraining natural killer cells

Landmark discovery brings researchers closer to vanquishing virus
June 12, 2017

Dr. James Carlyle (left), a senior scientist at Sunnybrook Research Institute, and his former PhD student, Dr. Oscar Aguilar, discovered a viral molecule that inhibits natural killer cells.
A group of doctors published a report in 1982 in the Journal of Pediatrics about a family ravaged by disease. The parents were healthy, but three of their four children had severe infections from the Epstein-Barr virus, a common virus that is part of the herpesvirus family. Many people carry it, but do not get sick; those who do typically have flu-like symptoms that resolve on their own. In this family, however, three siblings suffered dire bouts of mononucleosis, which was caused by the virus and required them to be hospitalized. Two of them died. Curiously, the fourth sibling had a mild case of mononucleosis but recovered fully. Blood tests enabled doctors to pinpoint the cause of devastation in the family’s sickest members: natural killer cell deficiency.
Natural killer (NK) cells are a type of white blood cell that fights cancer and infection. Their job is to patrol the body and help protect it from disease. To prevent an attack on healthy cells, however, NK cells are normally restrained. When needed, they spring into action by two mechanisms. One occurs when stimulatory receptors on NK cells bind to stimulatory molecules on tumour cells or cells infected with a virus. This interaction, called “induced-self” recognition, tells the NK cell to release a lethal protein that tells diseased cells to die. The other, which leads to the same outcome, is called “missing-self” recognition: NK cells sense a loss of self-recognition proteins on their surface that is caused by pathogens. The loss of these proteins acts as a signal that something foreign has invaded and unleashes the NK cells’ offensive, which is how the body fights infection.
Over time, viruses have found ways to bypass the body’s defences. This phenomenon has captured the attention of Dr. James Carlyle, a senior scientist at Sunnybrook Research Institute (SRI), whose study of mouse cytomegalovirus (CMV), a herpesvirus, highlights this struggle between host and pathogen. “We’re using the virus as a tool to tell us what’s important about the immune system. Since the virus has co-evolved with the host over millennia to find a way to get around the immune system, it’s telling us aspects about immunity we previously didn’t understand,” says Carlyle, who is also an associate professor of immunology at the University of Toronto.
He and Dr. Oscar Aguilar, who did his PhD training in Carlyle’s lab, have discovered a viral molecule that inhibits NK cells. This molecule, aptly called an immunoevasin, is also the first protein known to bind to the NK1.1 receptor, which belongs to the NKR-P1 family of NK cell receptors. The NK1.1 receptor was first discovered 40 years ago as the prototypical NK cell marker. Since then, researchers have tried to find any molecule that interacts with NK1.1, but have come up empty—until now. “We’ve identified a number of other receptor-ligand interactions, but this is the first ligand identified for the very first NK cell receptor discovered,” says Aguilar.
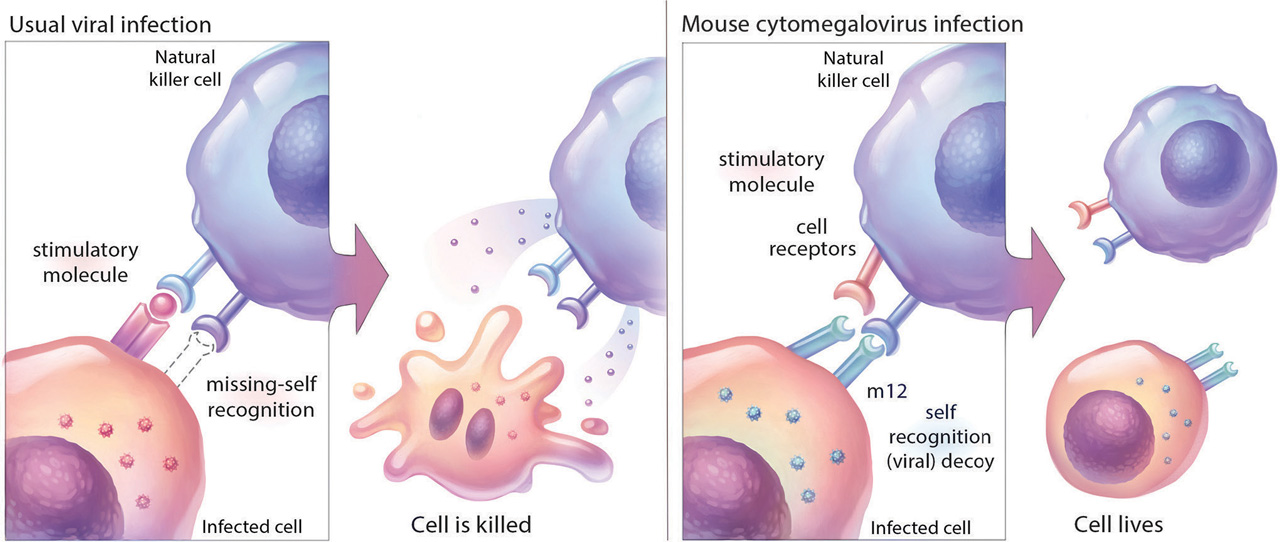
Left to right: A natural killer (NK) cell identifies a virally infected cell as “foreign” and kills it. Scientists at Sunnybrook Research Institute have
discovered, however, that cells infected with a mouse herpesvirus evade death by making a protein (m12) that restrains the NK cell.
Illustration: Hang Yu Lin
They published a study in Cell that shows m12, a viral protein identified in mouse CMV, binds to NK1.1 and other NKR-P1 receptors. This family of NK cell receptors has three stimulatory receptors that activate NK cells (including NK1.1) and two inhibitory receptors, which bind to inhibitory “self” molecules on target cells and shut down NK cells.When the researchers studied cells infected with mouse CMV, they observed decreases in a self-recognition protein called Clr-b—a “missing-self” response that is akin to triggering an alarm. Interestingly, they also saw a strong signal from one of the inhibitory receptors, NKR-P1B, which rapidly shuts the alarm off.
By using different strains of the virus and analyzing interactions between molecules, Aguilar was able to identify m12 as the “decoy” molecule that was blocking NK cells by binding to the NKR-P1B inhibitory receptor. He and Carlyle could appreciate the strength of this interaction thanks to the work of collaborators in Australia, who generated an X-ray crystal structure showing the binding of the two molecules. “It’s a very neat interaction,” says Aguilar, making a fist and wrapping his other hand around it. “The m12 [protein] binds like my hand on top, with a palm and polar-charged fingers that tightly bind to the NKR-P1B receptor. That is why our collaborators coined this a ‘polar claw’ mechanism.”
Aguilar, the study’s first author, notes the m12 protein is an example of how herpesviruses are suited to sidestepping the immune system, owing in part to their large genomes. “Some other viruses like HIV have much smaller [genomes] and don’t have the luxury of encoding a molecule like this,” he says.
Aguilar (left) and Carlyle published a study in Cell that shows m12, a viral protein identified in mouse herpesvirus, restrains natural killer cells.
What also surprised the researchers is that the m12 protein weakly interacted with two stimulatory receptors, called NKR-P1A and NKR-P1C (NK1.1). The results suggest an unfolding struggle, one where each organism is adapting to the other’s devices. “The weak stimulatory interaction really demonstrates the host and pathogen evolution,” says Aguilar. “The reason why the inhibitory interaction is the stronger response is because the virus is evolving to make sure it inhibits NK cells, whereas the host is trying to evolve mechanisms that also recognize this decoy—but I think the virus is winning, at least with respect to this [study].”
The next step is to study CMV and the NKR-P1A receptor in humans to see if there is a corresponding human viral decoy protein. About 70% of adults carry CMV, but most show no symptoms because a healthy immune system prevents the virus from causing illness. In people whose immune systems are compromised, however, like newborns, those with AIDS, or people who are undergoing chemotherapy or organ transplantation, CMV can cause serious damage, for example, to the liver, lung, eyes and nervous system.
If such a decoy is found in human CMV, then it could serve as a therapeutic target. Researchers could develop an antibody to the protein, which would give people with an immune deficiency like the one in the family profiled above, a fighting chance. “Using the monoclonal antibody to the viral protein [would] turn the immune response against the virus. Where [the virus] is trying to evade immunity, now it’s expressing a ‘flag’ that tells the immune system that the cell is infected and would result in more rapid clearance,” says Carlyle.
Aguilar was funded by the Natural Sciences and Engineering Research Council of Canada. Carlyle was funded by the Burroughs Wellcome Fund, Canada Foundation for Innovation, Canadian Institutes of Health Research, and Ontario Ministry of Research, Innovation and Science.



