Brain tumours
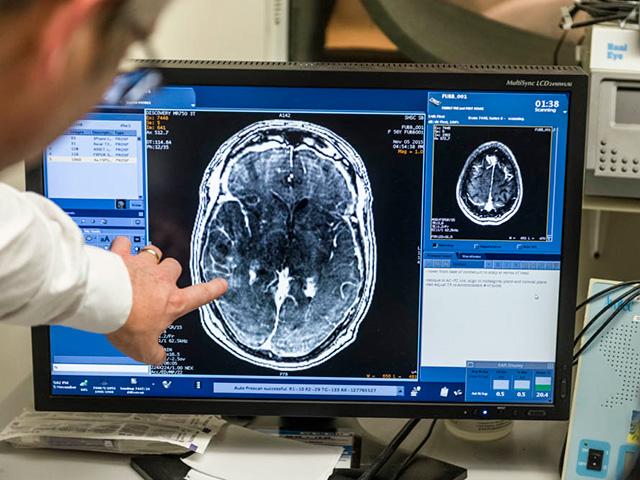
Magnetic resonance imaging enables precise application of ultrasound to the target. It also provides feedback during the procedure, allowing researchers to monitor blood-brain barrier opening and therapy delivery in real time.
Tumours located within the brain can be difficult, and sometimes impossible, to treat. Surgery is invasive and carries risks of complications. Drug therapies are ineffective because the blood-brain barrier blocks almost all drugs and therapies, including chemotherapies, from accessing the brain.
In 2015, a Sunnybrook team led by Dr. Kullervo Hynynen, director of Physical Sciences at Sunnybrook Research Institute (SRI), and neurosurgeon Dr. Todd Mainprize became the first in the world to use low-intensity focused ultrasound to breach the barrier and deliver chemotherapy into the brain of a patient with brain cancer. The study, published in January 2019 in Scientific Reports, showed that MRI-guided focused ultrasound can disrupt the blood-brain barrier safely and allow chemotherapy drugs to enter the brain.
The study is the culmination of decades of work for Hynynen. He was the first to show that focused ultrasound guided by MRI could be used to open the blood-brain barrier safely and reversibly. That it can be reversed is important, because it means the barrier can be closed again after therapy, thus keeping the brain safe.
During the procedure, patients are injected first with the chemotherapy drug, then with microscopic bubbles, directly into the bloodstream. Magnetic resonance imaging is used to direct beams of ultrasound to the site of the tumour. The sound waves cause the microbubbles to vibrate as they compress and expand. This motion loosens the tight connections between the cells that make up the blood-brain barrier, creating gaps through which the drug can enter.
Sunnybrook is also leading a clinical trial testing the safety and feasibility of MRI-guided focused ultrasound surgery for local treatment of progressive brain tumours in patients who have already received maximal surgical resection and radiation therapy. For more information, visit the clinicaltrials.gov website. If you are interested in participating in the trial, then please contact Allison Bethune, research manager, at 416-480-6100 ext. 64831.
In October 2018, Dr. Nir Lipsman, a scientist at SRI and a neurosurgeon at Sunnybrook, launched a clinical trial for glioblastoma. He is evaluating the safety, feasibility and effectiveness of using focused ultrasound to open the blood-brain barrier in people with glioblastoma who have had surgery, chemotherapy and radiation treatment, and who are receiving standard of care chemotherapy.
Glioblastoma is the most common and aggressive form of primary brain cancer. The cells of these tumours grow quickly and can spread throughout the brain. For more information about the study, visit the clinicaltrials.gov website: NCT03616860. If you are interested in participating in the trial, then please contact Maheleth Llinas, research manager, at 416-480-6100 ext. 62476.



