Blood not so simple

Preventing trauma patients from bleeding out is one of the most challenging tasks a trauma team faces—but so is ensuring they don't get lethal blood clots
June 12, 2017

Dr. Barto Nascimento and Dr. Jeannie Callum are leading studies in resuscitation and transfusion for trauma patients.
Ten minutes before our meeting, an email from Dr. Jeannie Callum pops up on my screen: “Multiple traumas right now. I won’t be able to talk—sorry.” Moments earlier a Code Omega call had been broadcast over the hospital’s intercom system. Someone was bleeding to death in the trauma bay, and Callum needed to be there.
After head injury, bleeding is the leading cause of death among trauma patients. “The estimate is that somewhere around half of [trauma] deaths are related to poor hemorrhage control,” says Callum, who is an associate scientist in the Tory Trauma Research Program at Sunnybrook Research Institute (SRI) and Sunnybrook’s director of transfusion medicine. Doctors must work quickly to replenish the fluids that have been lost and staunch the flow of blood to prevent patients from bleeding out. “On Friday [during the Code Omega], in a period of four hours, we issued 300 components of blood for a single patient,” she recalls.
The injuries and blood transfusions they receive, paradoxically, make these patients more prone to developing blood clots, or emboli, that could be lethal if they lodge in the lungs. “In the past, it was not uncommon for patients to die of a pulmonary embolism—where the clot dislodges from a vein in the leg and then prevents blood flow to the lung,” says Dr. Avery Nathens, director of the Tory Trauma Research Program at SRI and Sunnybrook’s surgeon-in-chief. “It’s always been a very challenging area because of balancing the risk between bleeding and clotting.”
Hemorrhage control and embolism prevention, then, are two sides of the same crimson coin. To give their patients the best chance of survival, Callum, Nathens and clinician-scientists at SRI are defining best practices that help physicians strike the right balance between these two seemingly opposite strategies.
In a test tube, blood looks like a homogenous, thick, dark red liquid, but it is actually a mixture of four main components: red blood cells that deliver oxygen; white blood cells that fight infection; platelets that clot at the site of injury; and liquid plasma that carries everything through the body.
Dr. James Byrne (left) and Dr. Avery Nathens published definitive results on how to prevent pulmonary embolism after trauma.
“When you lose blood, you lose everything,” says Dr. Barto Nascimento, an associate scientist in the Tory Trauma Research Program at SRI and a trauma hospitalist at Sunnybrook who specializes in the care of patients in hospital. “You’re bleeding red blood cells. You’re bleeding plasma, which has all the clotting factors, including platelets.” Before a transfusion, doctors typically rely on blood tests to determine the best ratio of blood components to mix together for each patient. Some may need more red blood cells; others, more plasma. The downside to this approach is that the tests take a long time. When a patient is bleeding out on the stretcher, each minute becomes as valuable as every drop of fluid dripping through an IV.
In an effort to start transfusions earlier, some doctors began using higher fixed ratios of blood products. The U.S. Army was the first to adopt so-called damage control resuscitation in its battlefield hospitals. Patients were automatically transfused with a balanced 1:1:1 ratio of plasma to platelets to red blood cells. Initial reports claimed that this strategy produced better outcomes than the traditional laboratory results-guided approach. These improvements were largely attributed to patients receiving a higher dose of plasma earlier in the equal ratio protocol.
“There was a problem with those studies,” says Callum at our rescheduled meeting five days later. “It wasn’t randomized. They had many patients who died before they had the opportunity to get plasma.” Without proper randomization, the sickest patients—those who succumbed within the hour it takes to prepare the plasma—were overrepresented in the control group, thus skewing the results in favour of the 1:1:1 approach.
To address the controversy, Callum and the Sunnybrook team took part in the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial, the first multicentre, randomized controlled trial (RCT) to examine the safety and effectiveness of the 1:1:1 protocol. The trial included 680 severely injured patients treated at 12 level I trauma centres in North America. The patients were randomly assigned to receive either a 1:1:1 mixture of plasma, platelets and red blood cells; or a 1:1:2 mixture, which is representative of what laboratory results typically recommend. There was no difference in the rate of death from all causes between the two groups at 24 hours and at 30 days after treatment. When the researchers drilled down into specific causes, however, they found that significantly fewer patients in the 1:1:1 group died from blood loss at 24 hours. Further, the balanced protocol stemmed the flow of blood in more patients compared with the 1:1:2 strategy. The results were published in the Journal of the American Medical Association in 2015.
At hospitals and blood banks, the higher ratio of plasma given in the 1:1:1 protocol raised concerns about safety and resource utilization. “There’s growing evidence of the severe complications of transfusing plasma,” says Nascimento, who is an assistant professor in the department of surgery at U of T. These adverse effects include transfusion-related acute lung injury, a rare but dangerous condition where patients go into respiratory distress after receiving blood products.
Using a fixed 1:1:1 strategy also means that thawed, ready-to-go plasma needs to be available all the time. Patients arriving at the hospital in need of a transfusion are initially given universal group O blood and type AB plasma until lab results confirm their blood type. As Nascimento notes, however, only 4% of blood donors are type AB, which means that blood banks experience a chronic shortage of AB plasma. Thawed plasma that is not used within five days must be thrown out, raising the possibility that the 1:1:1 approach could drain an already precious resource. The PROPPR trial allayed both fears—it found no difference in the number of transfusion-related complications between the two groups at 30 days, and a secondary analysis showed that the 1:1:1 protocol could be implemented without excessive wastage of AB plasma.
Pumping patients full of blood can buy physicians more time but does not address the root causes of bleeding—damage to blood vessels and clotting factor deficiencies. The former can be remedied with surgery and time; the latter requires a recalibration of the so-called coagulation cascade, a chain of biological processes that is set off upon vascular injury. An essential player in these events is fibrinogen, a clotting factor protein that works with platelets to plug leaky vessels. “Nowadays, we believe that fibrinogen is key for clotting,” says Nascimento. “Even if you don’t have a lot of platelets, if you have enough fibrinogen, you can clot.” In a patient that is hemorrhaging, though, it is the first clotting factor to drop to dangerous levels, making its supplementation a critical part of the resuscitation strategy.
Read text-only version of above infographic
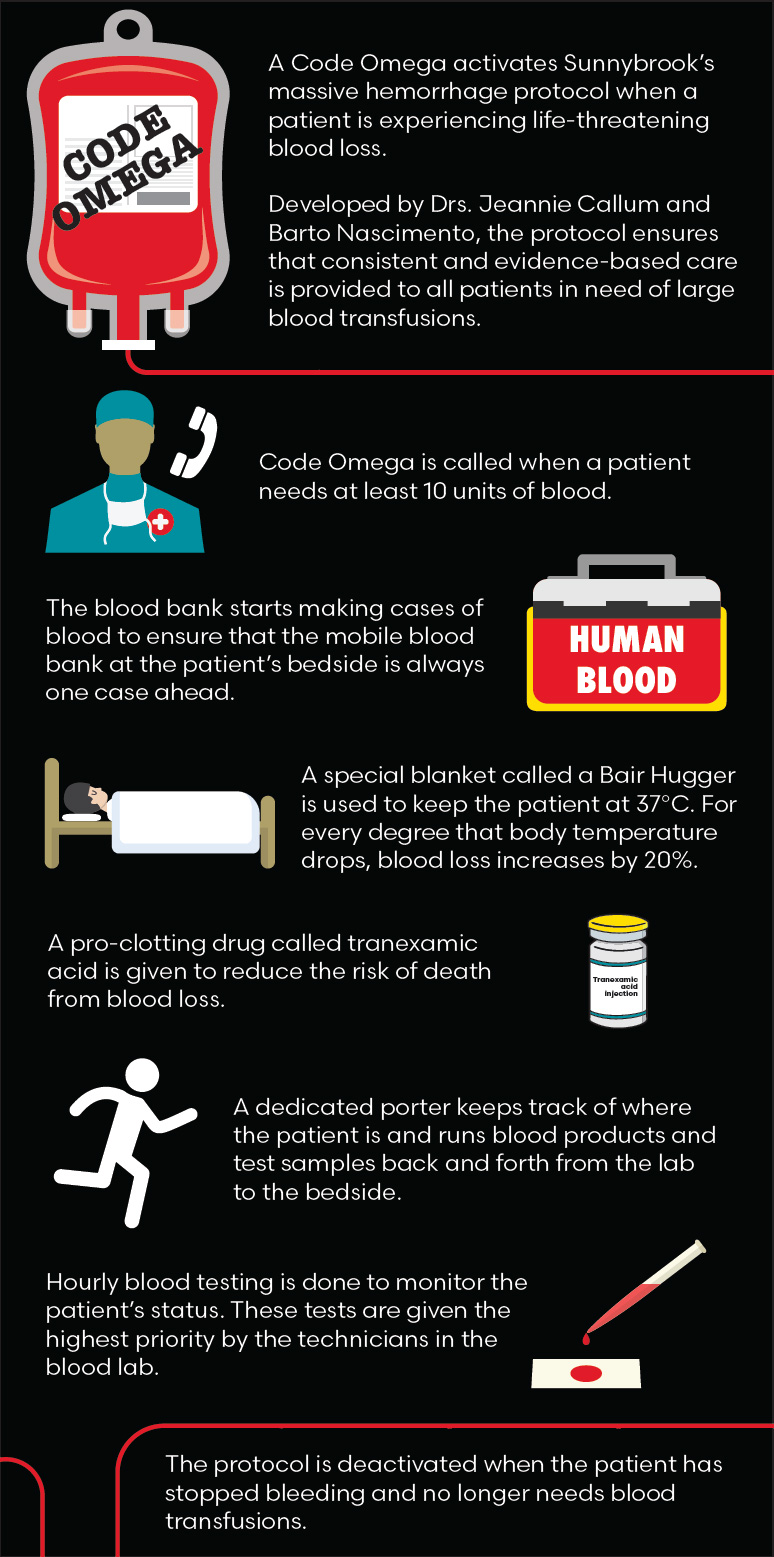
Code Omega
-
A Code Omega activates Sunnybrook's massive hemorrhage protocol when a patient is experiencing life-threatening blood loss.
-
Developed by Drs. Jeannie Callum and Barto Nascimento, the protocol ensures that consistent and evidence-based care is provided to all patients in need of large blood transfusions.
-
Code Omega is called when a patient needs at least 10 units of blood.
-
The blood bank starts making cases of blood to ensure that the mobile blood bank at the patient’s bedside is always one case ahead.
-
A special blanket called a Bair Hugger is used to keep the patient at 37°C. For every degree that body temperature drops, blood loss increases by 20%.
-
A pro-clotting drug called tranexamic acid is given to reduce the risk of death from blood loss.
-
A dedicated porter keeps track of where the patient is and runs blood products and test samples back and forth from the lab to the bedside.
-
Hourly blood testing is done to monitor the patient's status. These tests are given the highest priority by the technicians in the blood lab.
-
The protocol is deactivated when the patient has stopped bleeding and no longer needs blood transfusions.
In Europe, patients who are bleeding out receive fibrinogen concentrate, a dried and purified form of the protein that is dissolved in water before being infused into the patient. Here in North America, patients with low levels of the clotting factor receive cryoprecipitate, a frozen blood product derived from donated plasma. Unlike fibrinogen concentrate, the golden honey-coloured cryoprecipitate contains a mixture of fibrinogen and other clotting factors. More concerning, however, is that cryoprecipitate is not treated to kill any viruses that might be present in the original donor plasma.
“European governments decided that cryoprecipitate was not safe because it’s not virally inactivated,” says Callum, who is also an assistant professor in the department of laboratory medicine and pathobiology at U of T. “That obviously makes physicians in North America, the U.K. and Australia uncomfortable, because we’re still using cryoprecipitate when other countries have switched to blood products that have been deemed safer.”
For fibrinogen concentrate to become the standard of care in North America, evidence is needed to show that it can be administered quickly and that it is as safe and effective, if not more so, than cryoprecipitate. To that end, Nascimento and Callum led the first RCT to examine the feasibility and efficacy of using fibrinogen concentrate in trauma resuscitation.
“We were able to demonstrate that we could deliver this intervention very quickly at the bedside of trauma patients,” says Nascimento. Almost all—96%—of patients randomized to the fibrinogen concentrate group received the product within an hour of admittance to the hospital. For each patient, eligibility determination and randomization took 30 minutes, while the preparation and administration of fibrinogen concentrate took 30 minutes or less—far shorter than the time required to thaw, mix and deliver a dose of cryoprecipitate. The results were published in the British Journal of Anaesthesia in December 2016.
While it’s too early to say if the study will change practice, Nascimento is optimistic. “The coagulation system behaves better and patients are able to clot faster and stronger if fibrinogen is given early on,” he says. “We believe that this is an intervention that should be used routinely in the trauma clinic for patients.” The success of the trial has pushed him to run a large, multicentre RCT with Canadian trauma centres to determine whether fibrinogen concentrate can improve coagulation in patients and therefore decrease the need for blood transfusions. Then there is the economic upside. “We believe fibrinogen concentrate is also a blood conservation strategy that can be used to save money,” he says. He hopes that the data from this trial and similar studies being planned in Australia and the U.K. will persuade Canadian policymakers to license fibrinogen concentrate for use in trauma and shift practice toward a safer and more cost-effective product.
The blood transfusions and clotting factor supplementations that trauma patients receive prevent them from bleeding out; however, these life-saving measures also change the makeup of blood, such that it clots more easily. This trauma-induced hypercoagulopathy, along with vascular injury and abnormal blood flow due to immobility, put trauma patients squarely in the middle of Virchow’s triad, a set of three risk factors that contribute to the formation of blood clots. “Every single trauma patient is at high risk for developing a blood clot,” says Dr. James Byrne, a surgeon-scientist completing his PhD with Nathens. “Their prevention is a very important thing to address.”
Venous thromboembolism occurs when a blood clot forms in the vein. When the blockage is in a deep vein, such as those in the leg, the condition is called deep vein thrombosis (DVT). Occasionally, clots can break loose from other sites in the body and travel to the lungs, leading to a pulmonary embolism. While DVT occurs more commonly in trauma patients, pulmonary embolisms pose a greater risk of death because they can block some or all of the blood flow to the lungs and deprive the body of oxygen.
In the mid 1990s, Sunnybrook physician Dr. William Geerts became one of the first to describe the incidence of DVT in patients with trauma. He later conducted the first large RCT comparing the effectiveness of two types of the blood-thinning drug heparin in preventing DVT. “His was really a pivotal trial,” says Byrne. “He showed that low molecular weight heparin was associated with lower rates of DVT compared to unfractionated heparin.” Geerts’ seminal work led many hospitals, including Sunnybrook, to adopt low molecular weight heparin as the agent of choice for DVT prevention in trauma patients.
Despite the wealth of studies on how to avoid DVT, less is known about the best agent to thwart blockages in the lung, a more fatal complication. To address that gap, Byrne, Geerts and Nathens combed through data on more than 150,000 patients treated at 217 trauma centres enrolled in the American College of Surgeons Trauma Quality Improvement Program. “We wanted to compare the rate of pulmonary embolism between patients who received low molecular weight versus unfractionated heparin,” says Byrne. Unfractionated heparin is a blend of differently sized heparin molecules—some heavy, some light. In contrast, the low molecular weight agent is a purer concoction of lighter weight heparin molecules with greater potency and less risk of complications.
In their data set, low molecular weight heparin was given in 74% of cases. After matching for patient and hospital traits, the researchers found that the lower weight agent was associated with a significantly reduced rate of lung clots compared with unfractionated heparin. This pattern held true even when they compared patients who were given different prophylactic agents within the same hospital. “This study shows that the type of prophylaxis we use really does influence risk of pulmonary embolism,” says Byrne. These results, published in the Journal of Trauma and Acute Care Surgery in November 2016, were the first to show a relationship between the type of prevention agent and rate of lung embolism in patients with major trauma.
“There’s no question low molecular weight heparin is a better agent. All the RCTs have shown this,” says Nathens, who is also a professor of surgery at U of T. Despite data supporting use of low molecular weight heparin in preventing DVT and now pulmonary embolism, roughly one-third of American hospitals still use unfractionated heparin because of its lower cost. The same is not true in Canada, where nearly all hospitals use the lower weight agent. “I think this study pushes the evidence a little bit more to further favour low molecular weight heparin. It probably will lead to more hospitals converting,” says Nathens.

The trauma team leader—TTL—is the conductor of the trauma bay. A physician with expertise in resuscitation, he or she coordinates the actions of the doctors and other specialist health professionals caring for a trauma patient. A TTL can be an emergency medicine doctor, surgeon or anesthesiologist. Here, trauma surgeon Dr. Barto Nascimento works as TTL at Sunnybrook, which has nine such specialists.
Nathens and Byrne are also hoping that their work will persuade physicians to change the way they inhibit the formation of blood clots in another vulnerable patient population—those with traumatic brain injury. “One of the big dilemmas in venous thromboembolism prophylaxis in trauma patients is what to do with patients that have severe brain injuries with intracranial hemorrhage,” says Byrne. The neurosurgeons who treat these patients are often reluctant to prescribe blood thinners like heparin for fear that it will worsen bleeding in the brain. “Any potential to increase the amount of blood in the brain is risky,” says Nathens. “In patients with head injury, probably 30% to 40% receive no prophylaxis whatsoever.”
Recognizing the need for more evidence, the researchers looked at data from over 3,600 patients compiled through the American College of Surgeons Trauma Quality Improvement Program. The patients all had a severe traumatic brain injury and were given venous thromboembolism prophylaxis with either low molecular weight or unfractionated heparin. Forty-three per cent of patients received heparin early within 72 hours of hospitalization. The rates of both pulmonary embolism and DVT were nearly twofold lower in the early prophylaxis group than in those who received heparin after 72 hours. There was also no difference in the rate of late neurosurgical interventions or in-hospital mortality between the two groups, indicating that early administration of blood thinners did not worsen intracranial bleeding in these patients.
The results, published in the Journal of the American College of Surgeons in October 2016, were well received. “People welcomed this data because there’s so little out there,” says Nathens. By undertaking studies like this, he and his colleagues are helping to define best practices that can turn the crimson tide in the patient’s favour.
What was the state of blood clot prevention in trauma when you started your work?
The idea was these patients have a high risk of bleeding, and that risk stays high over time. If this were the case, then you would be reluctant to give an anticoagulant to someone whose risk of a blood clot was also high. Our hypothesis was that bleeding risk drops off. We thought that once the surgeon fixes the bleeding or it stops on its own, these patients should not be at high risk for bleeding. If we wait a little bit before we start anticoagulant prophylaxis in trauma patients, then we can prevent clots and not cause bleeding.
Why is the thromboembolism program at Sunnybrook unique?
Ever since the early 1990s [when the program started], we've seen every trauma patient that comes here. We give a bit of individualized prophylaxis to these patients depending on their risk of thrombosis and their risk of bleeding. That doesn't happen at other hospitals—no other trauma unit in the world has a thrombosis service that sees all their patients.
What has been the impact of your work?
Before we started, every year there was between one and six trauma patients who died of a pulmonary embolism (PE) at Sunnybrook. In the last 10 years, to the best of my knowledge, there's been one person who died of PE and that's with greatly increased numbers of trauma patients per year. I think what we do is the gold standard. Our experience here has had a huge impact on trauma care around the world.
Research Funding
Byrne: Canadian Institutes of Health Research (CIHR). Callum: Canadian Forces Health Services, CSL Behring, Defense Research and Development Canada (DRDC) and National Lung, Health and Blood Institute. Nascimento: DRDC. Nathens: CIHR and the De Souza Chair in Trauma Research.



