Next step in scalpel-free surgery
Sunnybrook researchers are initiating a clinical study to advance focused ultrasound, a form of scalpel-free surgery, to treat severe essential tremor, the most common movement disorder.
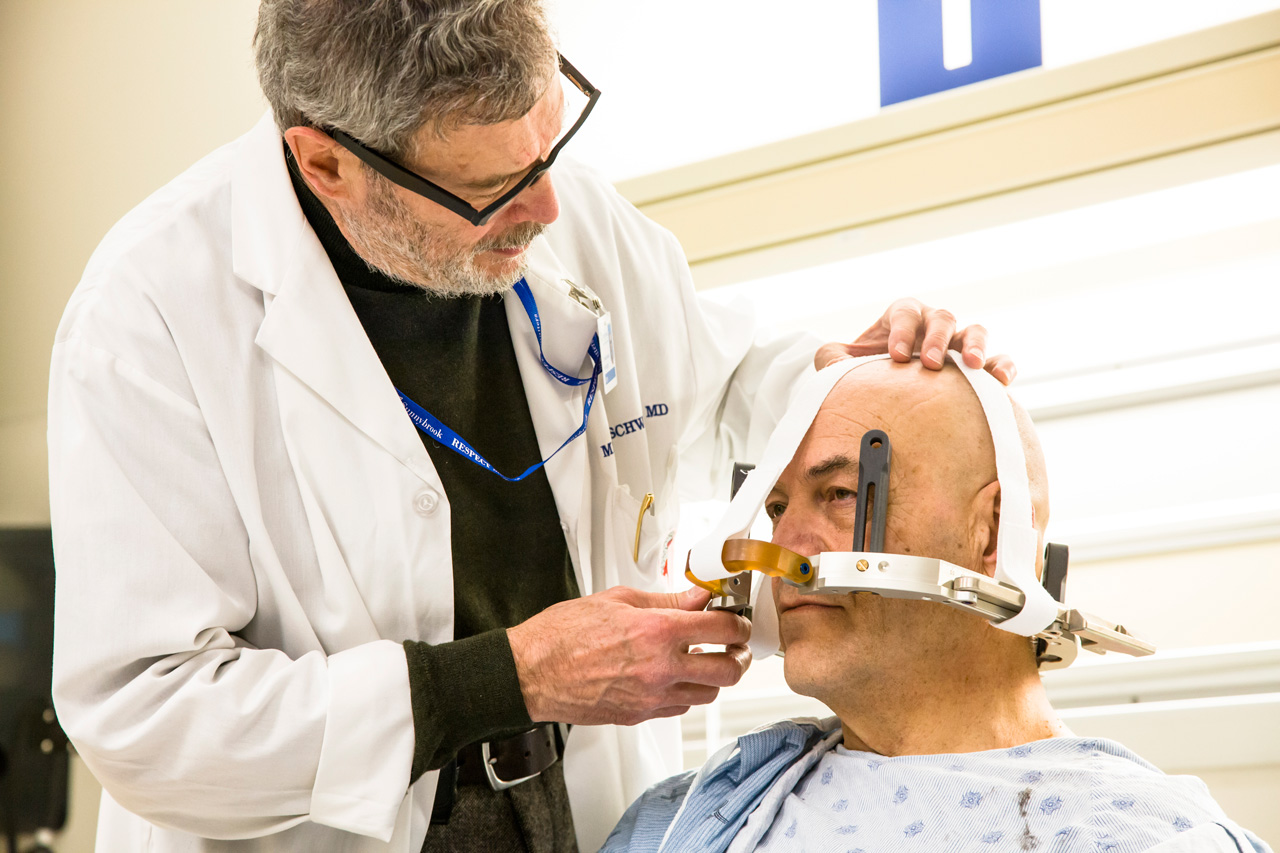
On March 3, 2014, Sunnybrook embarked on the start of a Phase III randomized clinical trial that is testing use of the ExAblate Neuro Focused Ultrasound System.
"We are excited about continuing the process to revolutionize medicine by taking this innovation to the next level for our patients," says Dr. Michael Schwartz, lead investigator of the Sunnybrook-site trial and neurosurgeon at Sunnybrook.
Dr. Schwartz also led an early-stage Phase I clinical trial of the therapy at Sunnybrook from 2012-2013 that received worldwide media attention.
In this current trial, 72 patients are being enrolled in up to eight centres around the world and randomized to either an ExAblate Neuro or sham (no) treatment.
The results of this multi-site trial are expected to support a submission of the system to Health Canada and the U.S. Food and Drug Administration for regulatory approval of focused ultrasound to treat patients with essential tremor.
The study builds on promising pilot studies, including the early-stage trial conducted at Sunnybrook, demonstrating the preliminary safety and effectiveness of MR-guided focused ultrasound technology in treating target areas deep inside the brain.
"This study is an important step toward providing a unique, non-invasive alternative treatment for patients who are severely disabled by essential tremor, a very common movement disorder," said Eyal Zadicario, Vice President of R&D and Director of InSightec's Neuro program. "The Phase I studies showed that patients experienced immediate and significant symptom improvement. We expect that the results of this Phase III trial will demonstrate long term durable improvement and open the door of this technology to additional applications in the brain."
MR-guided focused ultrasound treatment is suitable for people whose severe medication-resistant tremor interferes with activities of daily living like writing, eating and drinking from a cup.
"Focused ultrasound is an alternative to surgery with the potential for less risk of hemorrhage, infection and brain damage," said Dr. Neal Kassell, chairman of The Focused Ultrasound Foundation. "We hope this pivotal trial will demonstrate safety and long-term efficacy, leading to the regulatory approval of this non-invasive technology to help improve quality of life for people suffering from essential tremor."
Referrals for the Sunnybrook trial can be faxed to 416-480-6085. For more information and treatment centres, please go to clinicaltrials.gov (NCT01827904) and US.insightec.com
Watch a video of the December 2012 trial at Sunnybrook: