Personalizing therapies through imaging
Researchers at Sunnybrook Research Institute (SRI) have demonstrated for the first time the feasibility of using high-content imaging to bring precision medicine to patients with chronic lymphocytic leukemia (CLL), a type of blood cancer that originates in the bone marrow.
In a study published in the journal Blood, the research teams of SRI scientists Drs. David Andrews and David Spaner used cells from patients with CLL to understand how resistance to a drug called venetoclax that is prescribed for CLL develops, and how that resistance can be overcome. Dr. Sina Oppermann, a postdoctoral fellow in Andrews’ lab, led the study. Venetoclax works by blocking a protein called Bcl-2, whose abundance in CLL cells contributes to the cells’ cancerous properties. Andrews is interested in Bcl-2 because of its role in preventing apoptosis, a carefully controlled and crucial process, whereby damaged and stressed cells are destroyed to prevent further injury to the body. In cancer cells the signals for this process are often suppressed, leading to uncontrolled growth. Venetoclax restores the balance by silencing the silencer, thereby selectively killing cancer cells.
While 75% of patients respond to venetoclax, complete remissions are seen in only 20% of these patients. The ability of CLL cells to hide out in specialized “niches” in the body contributes to the development of resistance to the drug. “In the niche [the CLL cells] get signals that tell them to proliferate,” says Andrews, who is the director of Biological Sciences at SRI. “That signalling is also telling them to be drug resistant.” He surmised that shutting down the signalling could make the cells sensitive to venetoclax again.
To test their hypothesis, the researchers used methods pioneered by Spaner to recreate the niche environment in the lab with CLL cells donated by patients. They found that cells from most patients grown in this environment were resistant to venetoclax, whereas the drug rapidly killed cells in the control environment. They next tested 320 drugs that are known to inhibit different signalling pathways in the cell. Each of these drugs was given with venetoclax to identify combinations that could overcome the resistance seen in the niche-grown cells.
Using samples from 13 patients, they found that very few combinations worked well in multiple patients, highlighting the need for therapies tailored to individual patients. The signalling inhibitor that produced the strongest effect in the most patient samples was sunitinib, a drug used to treat kidney cancer. For some patients the combination of sunitinib and venetoclax killed dramatically more CLL cells compared to venetoclax alone. Because venetoclax inhibits only the Bcl-2 protein, cells can get around this by shutting down pro-apoptotic proteins or boosting production of other anti-apoptotic proteins to replace Bcl-2. Sunitinib works in a subset of patients—those whose cells rely on other anti-apoptotic proteins to bypass venetoclax’ effects—by shutting down the signals that enable the cell to make those proteins.
The researchers also analyzed the DNA of the patients to look for patterns and markers that might predict drug resistance. They found no correlation between a patient’s genetic profile and drug response. “The DNA tells you about the severity of disease, but it doesn’t tell you anything about drug susceptibility,” says Andrews, who holds the Canada Research Chair in Membrane Biogenesis.
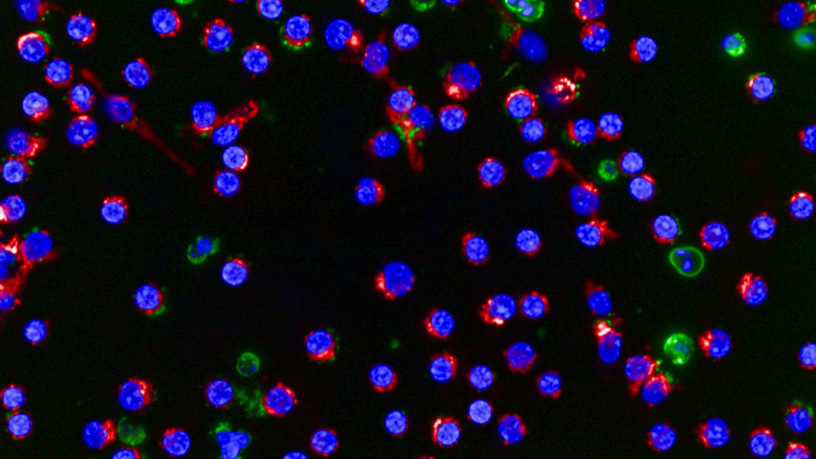
He notes that the success of this study relied on the powerful imaging capabilities of the Opera, an automated microscope capable of taking more than 100,000 images a day. His team also developed image analysis software that enabled computers to detect and calculate the number of dead and alive cells in a given image. With these capabilities, the researchers were able to test 320 different compounds in 640 combinations with and without venetoclax in just 96 hours, and identify which drug combinations might work best for an individual patient.
The promising results of this proof-of-principle study are paving the way for a clinical trial looking at the safety and effectiveness of sunitinib and venetoclax in treating patients with CLL. The researchers will try to validate their results in a new cohort of patients to see if results from lab-grown cells can predict how patients will respond in the clinic. If the trials are successful, then it would mean researchers could use this approach to identify which patients are likely to respond to venetoclax and sunitinib—before any potentially toxic or ineffective drugs are given. Ultimately, “the screening system can be used to predict the most appropriate drugs and drug combinations for individual patients,” says Spaner. “In the future, we might be able to personalize the treatment regimen by prescribing venetoclax along with the most effective [signalling] inhibitor identified from the screen.”
This study was funded by the Canada Research Chairs program, Canadian Institutes of Health Research, and Leukemia and Lymphoma Society of Canada. Infrastructure support comes from the Canada Foundation for Innovation.