Study of Treatment Approaches for Sleep Apnea
If you are 55 or older and have concerns about your sleep and your cognition (memory and thinking), then you may be eligible to participate in a clinical trial seeking to determine the best way to treat sleep apnea in order to improve memory and prevent the brain changes that lead to memory loss.
Participants in this trial will receive free access to cutting-edge web-based sleep education, free continuous positive airway pressure (CPAP) equipment to use during the study and to keep at the end of the study, free in-home sleep measurements using wearable sensors with feedback provided, free access to a personal sleep coach and visits with a sleep physician specializing in helping older adults get better sleep.
Sleep apnea, one of the most common causes of sleep difficulties in adults, is characterized by interruptions in breathing during sleep. People with sleep apnea have worse memory and concentration and they have a higher risk of developing Alzheimer’s disease and other causes of memory loss.
Facts about sleep apnea and the brain
- Sleep apnea is common, affecting over 30% of adults over the age of 60.
- Although many people with sleep apnea snore, some do not. Some people simply have unexplained difficulty staying asleep at night, problems with daytime fatigue, or problems with memory and concentration.
- Adults with sleep apnea have an almost 2x risk of developing cognitive impairment or dementia.
- Sleep apnea is easily treated by CPAP in combination with support for healthy sleep habits, like sleep education or sleep coaching.
Treating sleep apnea
Sleep apnea is effectively treated using CPAP – a simple mask, often just over the nose, that delivers extra air into your upper airway and lungs to support breathing – in combination with sleep education. This trial aims to determine the best way to treat sleep apnea: Is it more effective to treat sleep apnea with CPAP and sleep education simultaneously, or is it better to “set the stage” with sleep education before CPAP? This trial will answer this question in adults with mild cognitive impairment (MCI) and sleep apnea. MCI is characterized by memory and thinking problems beyond what is expected for your age, but not significant enough to interfere with activities of daily living. Over 75% of adults with MCI are able to use CPAP consistently, and those who do have a slower rate of cognitive decline than those who do not.
Participating in this trial
If you choose to participate in this trial, you will have 5 visits to Sunnybrook over 8 months as outlined in the figure below.
- Visit 1 is the screening visit. In order to be eligible for this trial, you will need to test positive for MCI through a memory and cognitive assessment and sleep apnea through simple sensors sent to you to wear for 1 night in your own home, followed by an overnight sleep study at Sunnybrook to confirm.
- Visit 2 is scheduled if you meet the screening criteria where you will be connected with a Sunnybrook sleep physician and a personal sleep coach. After this visit, you will be randomized into of two study groups: 1) simultaneous web-based sleep education program designed to support healthy sleep habits and CPAP for 8 months, or 2) sequential sleep education and CPAP, where you first receive 4 months of sleep education to “set the stage” for CPAP to begin at the beginning of month 5. All treatment will be free of charge and you will get to keep the CPAP equipment after the study.
- Visits 3-5 are assessment visits to allow us to determine which approach to managing sleep apnea better improves memory and prevents brain changes that lead to memory loss.
Click through the interative timeline below to learn more about what is required at each visit.
-
Screening
1-hour video-conference to test for mild cognitive impairment (MCI)
1 home sleep apnea test
1 overnight sleep study at Sunnybrook to confirm sleep apnea
-
Sleep Physician
1 hour at Sunnybrook to discuss screening results with sleep physician
Randomization to one of two groups:
- Simultaenous education + CPAP
- Sequential education + CPAP
-
Month 0
3 hours at Sunnybrook
Memory, concentration, and mood tests
Brain MRI + blood draw
Home sleep + activity measurements
-
Month 4
3 hours at Sunnybrook
Repeat Visit 3
-
Month 8
2 hours at Sunnybrook
Repeat Visit 3 without MRI
About
Why is this study being done?
We are trying to determine if CPAP and sleep education can slow the negative effects of disordered sleep and if it is more effective to treat obstructive sleep apnea (OSA) in adults with MCI with CPAP and sleep education simultaneously or to “set the stage” with sleep education before CPAP.
Obstructive sleep apnea (OSA)
Of particular interest to this study is a form of sleep disruption called obstructive sleep apnea; a disorder characterized by repetitive interruption of breathing in sleep. OSA is seen in approximately 50% of people with mild cognitive impairment.
Mild cognitive impairment (MCI)
MCI is characterized by cognitive impairment (memory and thinking problems) beyond what is expected based on participant’s age, but not significant enough to interfere with activities of daily living.
This study aims to provide evidence that treating sleep apnea with CPAP and sleep education can improve cognition and stop or slow the negative effects of disordered sleep, and to determine how best to combine these approaches.Study investigators
 Dr. Andrew Lim is a neurologist at Sunnybrook Health Sciences Centre in Toronto with a practice focused on helping older adults with and without cognitive impairment to get better sleep. He completed an MD and residency in neurology at the University of Toronto, a clinical sleep fellowship at the Beth Israel Deaconess Medical Center in Boston and a Master’s Degree in clinical investigation at Harvard Medical School. Dr. Lim’s research is focused on understanding how sleep disruption, including sleep apnea, leads to dementia and how dementia in turn worsens sleep.
Dr. Andrew Lim is a neurologist at Sunnybrook Health Sciences Centre in Toronto with a practice focused on helping older adults with and without cognitive impairment to get better sleep. He completed an MD and residency in neurology at the University of Toronto, a clinical sleep fellowship at the Beth Israel Deaconess Medical Center in Boston and a Master’s Degree in clinical investigation at Harvard Medical School. Dr. Lim’s research is focused on understanding how sleep disruption, including sleep apnea, leads to dementia and how dementia in turn worsens sleep.
 Dr. Brian J. Murray is the head of the division of neurology at Sunnybrook Health Sciences Centre. Dr. Murray's major research interests are in neurological aspects of sleep and the relationship between sleep and behaviour. Some clinical conditions of particular interest include narcolepsy, rapid eye movement sleep behaviour disorder, restless legs syndrome and the effects of sleep and sleep disorders in other medical conditions.
Dr. Brian J. Murray is the head of the division of neurology at Sunnybrook Health Sciences Centre. Dr. Murray's major research interests are in neurological aspects of sleep and the relationship between sleep and behaviour. Some clinical conditions of particular interest include narcolepsy, rapid eye movement sleep behaviour disorder, restless legs syndrome and the effects of sleep and sleep disorders in other medical conditions.
 Dr. Mark Boulos is a neurologist at Sunnybrook Health Sciences Centre. His research focuses on the association of sleep disorders with transient ischemic attack (TIA), stroke, dementia/cognitive impairment, and other neurological disorders. He is also interested in the neurophysiological aspects of sleep, home and in-hospital sleep monitoring, and developing novel approaches to manage sleep disorders.
Dr. Mark Boulos is a neurologist at Sunnybrook Health Sciences Centre. His research focuses on the association of sleep disorders with transient ischemic attack (TIA), stroke, dementia/cognitive impairment, and other neurological disorders. He is also interested in the neurophysiological aspects of sleep, home and in-hospital sleep monitoring, and developing novel approaches to manage sleep disorders.
This study is generously funded by: Weston Family Foundation

To indicate your interest in participating, or to request more information, please fill out this short survey and a member of the study team will be in touch. Thank you for considering participation in this important research study – we look forward to hearing from you!
Study intervention
Continuous positive airway pressure (CPAP)
The standard or usual treatment for OSA is CPAP. CPAP is a system comprised of a facemask connected by tubing to a machine that applies positive pressure to keep the upper airway open during sleep. In standard clinical pathways in Ontario, it can take approximately 4 months to receive CPAP from the time of diagnosis.
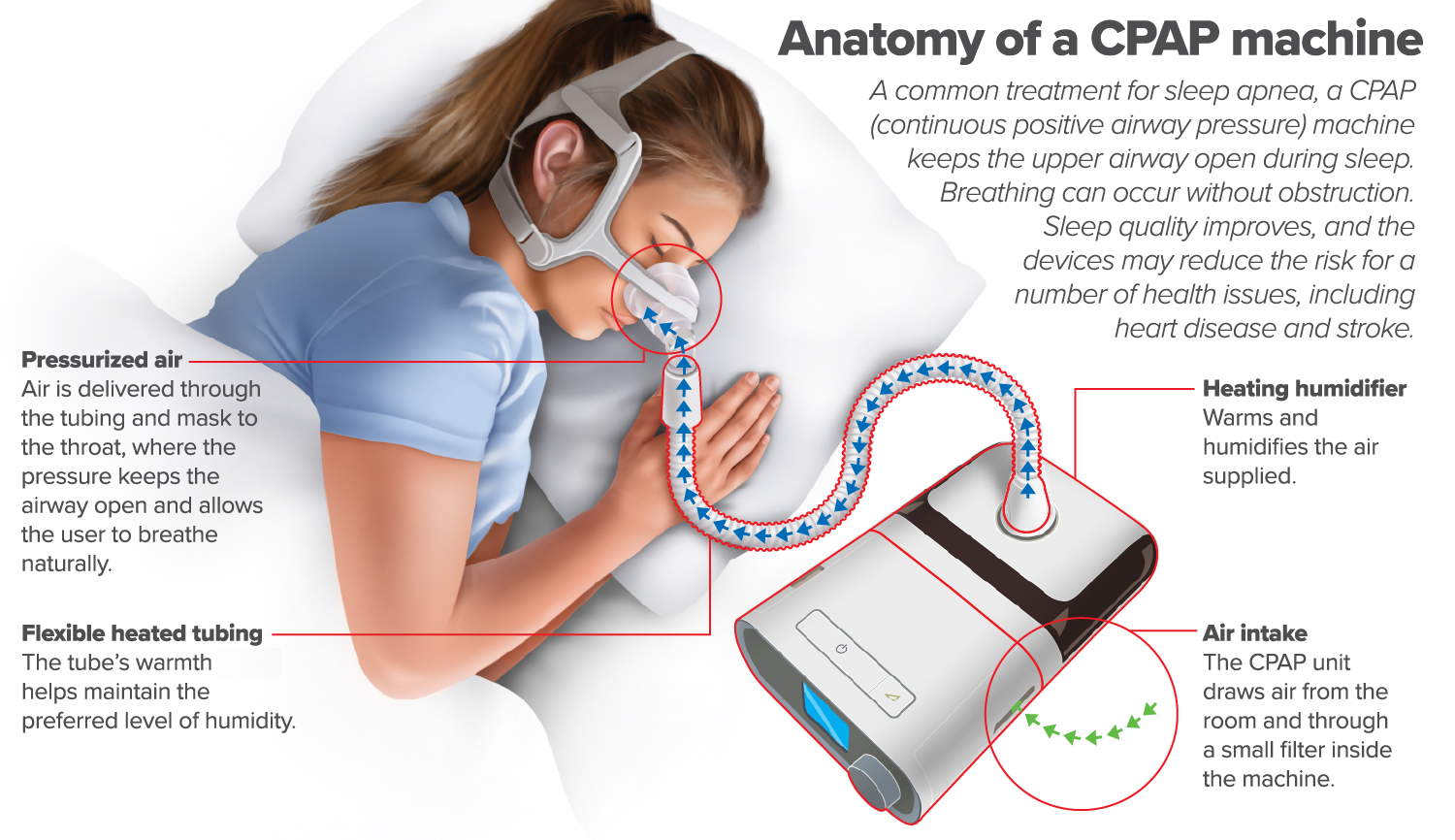
Click here to read a text-only version of this infographic
Anatomy of a CPAP machine
A common treatment for sleep apnea, a CPAP (Continuous positive airway pressure) machine keeps the upper airway open during sleep. Breathing can occur without obstruction. Sleep quality improves, and the devices may reduce the risk for a number of health issues, including heat disease and stroke.
Pressurized air
Air is delivered through the tubing and mask to the throat, where the pressure keeps the airway open and allows the user to breathe naturally.
Flexible heated tubing
The tube's warmth helps maintain the preferred level of humidity
Heating humidifier
Warms and humidifies the air supplied.
Air intake
The CPAP unit draws air from the room and through a small filter inside the machine.
Web-based sleep education
In addition to CPAP, web-based educational interventions are designed to convey best available evidence for healthy sleep that can slow cognitive decline and reduce AD-related brain changes. This study utilizes the Brain Health Pro (BHP) platform. BHP is a formal educational program designed to increase dementia literacy, foster engagement, and convey best available evidence for lifestyle changes that can mitigate dementia risk. The program content consists of 24 chapters that participants can read at their own pace and will be given the opportunity to discuss with research staff at scheduled check-in calls.
Which intervention will I receive?
The answer is – BOTH! But at different timings. Participants will undergo randomization to one of two groups with one group receiving CPAP and web-based sleep education at the same time and the second group receiving sleep education first to set the stage, followed by CPAP 4 months later.
Group 1: Simultaneous sleep education + CPAP
The simultaneous CPAP + education group will start CPAP at the beginning of month 0 of this study (to be used nightly from months 0-8) at the same time as participation in a web-based sleep education program.
Group 2: Sequential sleep education + CPAP
The sequential group will first have 4 months of sleep education to “set the stage” and then start CPAP after this (to be used nightly from months 5-8).
All participants will receive CPAP supported by a trained sleep technologist and web-based sleep education, all paid for by the study.
Both groups will undergo an in-person mask fitting and will receive support by a study sleep technologist with extensive clinical experience with CPAP. Masks, tubing, chin straps, and other equipment necessary to optimize CPAP adherence will be provided by the study. You will be asked to use CPAP nightly during your participation in this study for 4 months or 8 months, depending on which group you are in.
To indicate your interest in participating, or to request more information, please fill out this short survey and a member of the study team will be in touch. Thank you for considering participation in this important research study – we look forward to hearing from you!How to participate
To participate in this study, you must meet the inclusion criteria found below.
Inclusion criteria
- You are > 55 years of age
- You have had no drowsiness-related driving accidents or near misses in the past 12 months
- You do not drive as your primary occupation
- You are able to complete cognitive assessments in English (read, write, and understand)
- You are able to participate in video-based cognitive assessments
- You are a resident of Ontario
- You have no contraindications to MRI or reasons to think you cannot have an MRI
- You have not been treated for sleep apnea in the past
- You are able and willing to start CPAP now if you screen positive for sleep apnea
In addition to the above, we will screen you for MCI (video-based cognitive assessment) and sleep apnea (at-home test and an overnight sleep study at Sunnybrook, arranged by study staff). You will need to screen positive for both to be eligible for this study. We will also screen you for insomnia, restless legs syndrome, and ask you about any medications you are taking related to MCI or sleep (video-based screening).
Benefits of participating
All participants in this study will receive regular visits with a sleep physician at Sunnybrook specializing in sleep problems in older adults, access to a personal sleep coach, along with access to a cutting-edge web-based sleep education for 8 months that they would not otherwise receive as part of usual clinical care. The sleep education program used in this study was developed by the Canadian Consortium for Neurodegeneration and Aging (CCNA) aimed at optimizing sleep habits for dementia prevention.
All participants, regardless of randomization, will receive CPAP paid for by the study, no later than would have been possible through regular clinical pathways. This includes the necessary masks, tubing, chin straps, and other equipment to optimize CPAP adherence. While using CPAP in this study, you will be supported by a personal sleep coach as well as access to a sleep technologist with extensive clinical experience with CPAP. At the end of your participation, you will get to keep the CPAP device and equipment obtained throughout your participation. The study doctor can help you identify standard CPAP support available in community clinics at the conclusion of your participation in the study.
We hope the information learned from this study will help other people with MCI and sleep apnea in the future. This study could support widespread treatment of sleep apnea in patients with MCI to slow cognitive decline and reduce AD-related brain changes, and identify how best to combine treatments (i.e. sleep education, CPAP) to achieve this.
How do I sign up?
To indicate your interest in participating, or to request more information, please fill out this short survey and a member of the study team will be in touch. Thank you for considering participation in this important research study – we look forward to hearing from you!Study visits
The following tests will be done as part of this study:
Visit 1: Screening
- 1 hour video call to screen for eligibility: We will collect basic demographic and medical information; questionnaires about your sleep and sleep disorders including sleep apnea, insomnia, restless legs, and REM sleep behaviour disorder; tests of cognitive function, as well as an assessment of your daily functioning.
- 1 home sleep apnea test to test for sleep apnea: We will mail you a device to wear for 1 night of sleep.
- 1 overnight sleep study at Sunnybrook to confirm sleep apnea: We will schedule you for a diagnostic polysomnography (PSG) to confirm sleep apnea diagnosis.
Visit 2: Meet with Sunnybrook sleep physician
- 1 hour in-person visit with a Sunnybrook sleep physician to confirm diagnoses and address sleep difficulties
Visit 3: Baseline
- 3 hour in-person visit to Sunnybrook to complete an MRI, provide a blood sample, and complete some tests of your memory, concentration, and mood.
An MRI is a test that uses a magnetic field and radio waves to create detailed pictures of the brain. MRI is a safe and non-invasive diagnostic imaging test. The scan involves lying still on a bed that moves into the centre of the main magnetic field. MRI technologists at Sunnybrook will execute all MRI scans and are trained to address participant needs and maximize comfort. You will be able to communicate with the MRI technologists and study investigators at all times.
Blood samples will be taken by inserting a needle into a vein in your arm by study staff trained in blood draws. These samples serve several purposes. 1) Samples will be tested for various fats, sugars, and proteins (like cholesterol or glucose). 2) Samples will be sent to the laboratory of a co-investigator on this study at University Health Network (UHN) where they will be examined for the purposes outlined in this research protocol only. 3) Samples will be frozen and stored for future analysis in future studies related to sleep, cognitive impairment, and dementia, which may include testing for as-of-now unspecified genetic markers of health outcomes or other physiological traits. Under no circumstances will your blood samples be used for commercial use.
The tests of your memory, concentration, and mood are various paper/pen and computer tasks.
Visit 4: Month 4
- 3 hour in-person visit to Sunnybrook to repeat all tests from the baseline visit
Visit 5: Month 8
- 2 hour in-person visit to Sunnybrook to repeat all tests from the baseline visit, except the MRI. The MRI is done at baseline and month 4 only.
Compensation for study visits
To offset the costs of participating (for example: parking, fuel, transportation, meals), you will receive compensation that varies depending on the extent of study participation, as follows:
- Home sleep apnea testing: $10
- Visit 1-5: $50 each
Study documents
Download the consent form. You do not need to print or sign the consent form at this time. The consent form is included here just for your information.
Click on any study procedure below to download instructions. You do not need to print these documents, they will be provided to you when the time comes. These instructions are included here just for your information.
Download the MRI contraindication form. You will be asked to fill out this form prior to study visit 1 (baseline) and visit 2 (4-month).Contact us
To directly contact the study coordinator:
Phone: 1-647-608-9833 (available Monday-Friday, 8:30am to 4:30pm)
Email: andrew.centen@sunnybrook.ca
To indicate your interest in participating, or to request more information:
Fill out this short survey and a member of the study team will be in touch.
Address of Study Visits
Sunnybrook Health Sciences Centre
2075 Bayview Avenue
Toronto, ON M4N 3M5
Thank you for considering participation in this important research study – we look forward to hearing from you!Frequently asked questions (FAQ)
How many people will participate in this study?
What if the screening shows I do not have MCI or sleep apnea?
Are there any costs to participating? Is there any compensation for participating?
There are no costs associated with participating, however you may incur costs associated with transportation and parking. To offset these costs, you will receive compensation that varies depending on the extent of study participation, as follows:
- Home sleep apnea testing: $10
- Study visits 1-5: $50 each
Will I get to continue the intervention after the study has finished?
Are there any risks involved in participating in this study?
Risks and side effects related to CPAP are minor and may include:
- Congestion or runny nose
- Dry mouth, nostrils, eyes
- Facial pain or skin irritation
- Claustrophobia
Risks and side effects related to other study procedures are minor and may include:
- Discomfort or skin irritation from wearing some of the monitoring equipment, or minor disturbances of your usual sleep while wearing the monitoring equipment.
- Bruising, pain, or very rarely, loss of consciousness during blood collection
- Rarely, people experience temporary and mild tingling sensation during the MRI. You may also experience temporary stress/anxiety or claustrophobia during the MRI.


