New metabolic imaging helps better tailor chemotherapy
For the first time ever, Sunnybrook researchers demonstrate in as early as four weeks, breast cancer tumour response during patients' neoadjuvant chemotherapy using diffuse optical spectroscopy, a less expensive, "metabolic activity" detecting imaging modality. The study is published in Clinical Cancer Research and tracks responses over multiple times during patient therapy.
"We now have evidence at multiple times during pre-surgical chemotherapy that shows significant differences between patients responding to therapy and those who were not at four weeks after starting therapy," says Dr. Gregory Czarnota, principal investigator and radiation oncologist at Sunnybrook's Odette Cancer Centre and assistant professor, departments of Radiation Oncology and Medical Biophysics, University of Toronto.
"Our goal is to continue to reduce toxicity for women while ensuring the chemotherapy is optimal in reducing the tumour. With conventional imaging it is many months of chemotherapy before one might better tell how a patient is responding and whether to alter the regimen or the treatment approach. With this new, more functional imaging, we can see more tumour activity early on which means a better therapeutic ratio and better chance there will be no cancer after surgery," says Dr. Rebecca Dent, study co-investigator and medical oncologist and co-lead, Locally Advanced Breast Cancer Clinic at Sunnybrook's Odette Cancer Centre.
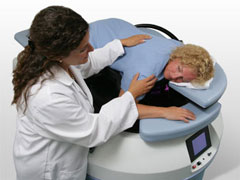
Diffuse optical spectroscopy shows strong potential over conventional imaging, providing functional imaging to better monitor tumour activity such as changes in oxyhemoglobin, deoxyhemoglobin, lipid and water content. This imaging modality uses risk-free near infrared light and does not require the patient to be injected with a contrast agent. It is relatively less expensive than modalities such as MRI and PET, and can be used more readily in smaller settings.
The device used to conduct the study is the SoftScan system developed by ART Advanced Research Technologies Inc., Montreal, Canada.
PDF / View full media release »