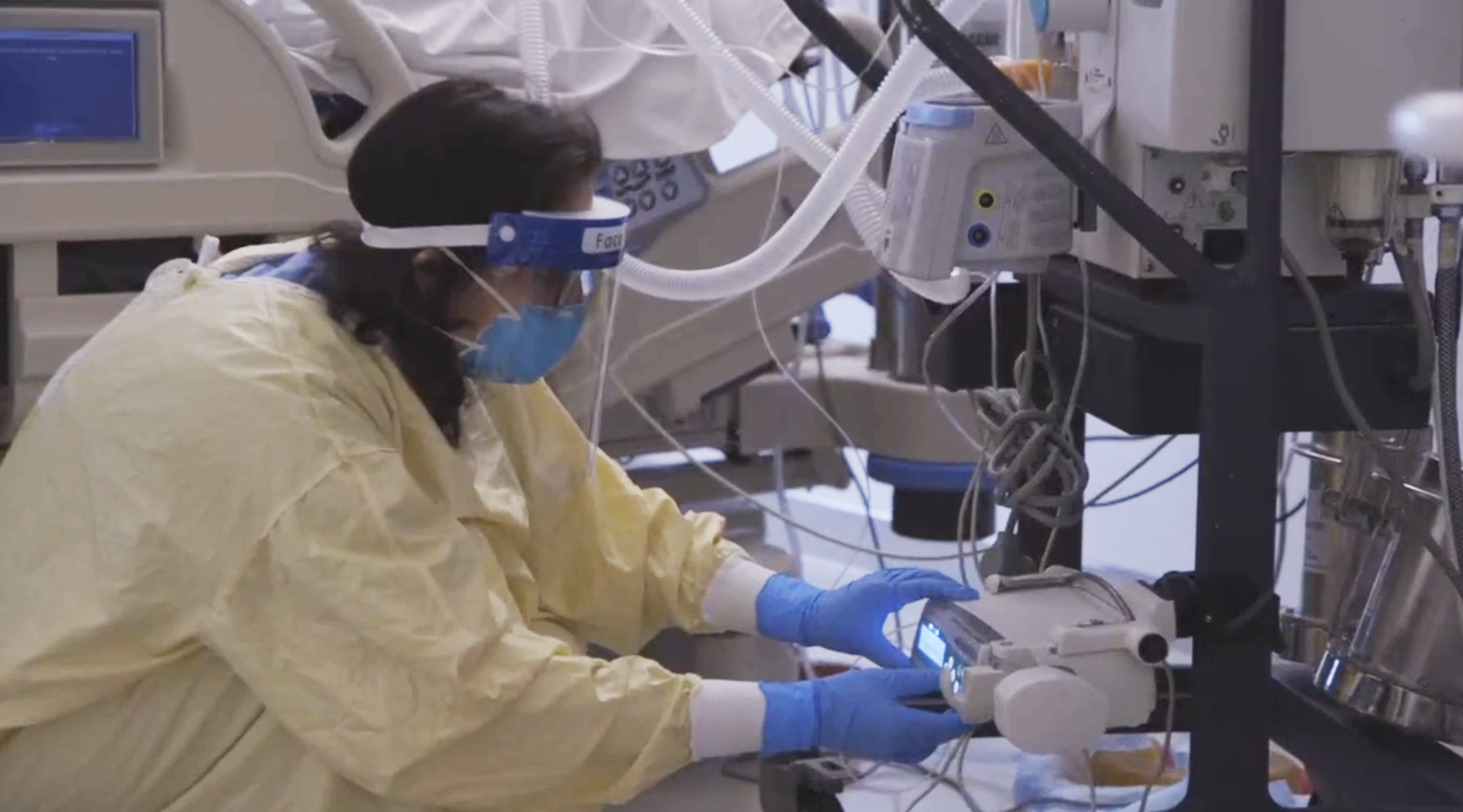
Sunnybrook researchers are trialing an alternative method of sedation for critically ill COVID-19 patients
A team of researchers from Sunnybrook Research Institute and Lawson Health Research Institute is leading a multi-centre trial investigating whether inhaled sedatives can replace those typically delivered intravenously for COVID-19 patients requiring ventilation.
“In order to tolerate the uncomfortable procedure of being put on a breathing machine, patients require intravenous (IV) sedation or sleep-inducing medications,” says Dr. Angela Jerath, an anesthesiologist and scientist in the Schulich Heart Program at Sunnybrook and the study’s lead principal investigator. “At the beginning of the pandemic, these drugs were in short supply due to the high number of patients needing ventilators.”
With a large number of patients with COVID-19 requiring ventilation in the third wave of the pandemic, especially younger patients, the research question is timelier than ever.
The clinical trial, a collaborative effort of researchers from across Canada, is comparing inhaled volatiles — a cheaper and more widely available anesthetic that is commonly used in operating rooms — versus the standard of care (IV sedation). “In addition to easing the pressure on IV sedation stocks, there is some evidence to suggest that these drugs may also have properties that reduce lung inflammation, which may speed up recovery and reduce the time patients spend on a ventilator,” says Dr. Jerath.
The study, the largest trial of its kind, is currently recruiting patients across 12 sites in Canada and in total will enroll approximately 750 patients, randomized to receive either IV or inhaled sedatives. Patient outcomes such as length of ventilation and survival will be compared between the two groups.
Sunnybrook critical care units successfully introduced this new sedation care pathway within six weeks aided by intensivists Dr. Brian Cuthbertson (co-principal investigator), Dr. Martin Chapman and Dr. Damon Scales (co-investigators), and supported by pharmacy, respiratory therapy, critical care nursing and anesthesia assistants.
“If inhaled sedatives are effective at shortening the duration of ventilation, we can potentially reduce the pandemic’s strain on ventilator capacity while also improving patient outcomes,” says Dr. Jerath. “If successful, the results of this trial could also result in a significant paradigm shift in how we sedate critically ill patients in ICUs around the world.”
This study is funded with support from the Canadian Institutes of Health Research (CIHR) COVID-19 Rapid Research Funding Opportunity and the Government of Ontario’s COVID-19 Rapid Research Fund.
Early in the pandemic, IV sedatives were in short supply and Sunnybrook researchers quickly implemented a new sedation method for critically ill COVID-19 patients.
— Sunnybrook Health Sciences Centre (@Sunnybrook) May 20, 2021
Now, 12 sites are trialing the approach, which may improve patient outcomes: https://t.co/iAyrrprL9t #CTD2021 pic.twitter.com/eHgcTwSGlz
Read more about the trial: Behind the research: Implementing practice change amidst a pandemic
Learn more about participation in this trial
Media Contact:
Samantha Sexton
Communications Advisor
samantha.sexton@sunnybrook.ca