Scientist discovers T cell development starts in the bone marrow
A team led by Dr. Juan Carlos Zúñiga-Pflücker, a senior scientist in Biological Sciences at Sunnybrook Research Institute (SRI), has shown that T cells, which are critical to healthy immunity, start to develop in the bone marrow. This flips knowledge of T cell development on its head, as, until now, the process was thought to begin in the thymus. The discovery could help to accelerate the development of improved treatments for diseases involving the immune system. The findings are outlined in a paper, published in the November 2019 issue of Nature Immunology.
To uncover this, Zúñiga-Pflücker’s lab—including the study’s co-first authors Drs. Edward Chen and Patrycja Thompson—created a mouse model that allowed them to turn off and on Notch, a signaling pathway that dictates whether a cell becomes a T cell or takes on a different cell fate. This in itself was cause for celebration, as no previous model had allowed researchers to control Notch this way.
The team found that when Notch was off, the cells they examined in the thymus, called thymus-seeding progenitors (TSPs), could not become T cells. This indicated that the decision to become a T cell was made before TSPs reached the thymus.
Zúñiga-Pflücker, who is also the chair of and a professor in the department of immunology at the University of Toronto, says he was shocked by the results. He notes the original aim was to understand better the role of Notch signaling and how T cells develop in the thymus—not to show that development initiates elsewhere.
“We began the experiment thinking what was known was in fact the case, that the cells in the thymus are the ones that want to be T cells,” he says. “In fact, they needed [the Notch signal] before entry, which is the surprising part.”
Thompson, who did her PhD training at SRI with Zúñiga-Pflücker, was similarly taken aback. “It was definitely surprising … . A number of research groups have suggested that T cell specification takes place in the bone marrow before the progenitors [or early cells] enter the thymus; but, with our approach, we were finally able to directly prove this phenomenon,” she says.
The team came to their realization when they toggled the Notch signal off and on in a mouse model created by Thompson during her PhD. To achieve this effect, they tinkered with a gene called RBPJ, “the lynchpin of the Notch signaling pathway,” Zúñiga-Pflücker says. They believed when Notch was activated via RBPJ, the cells in the thymus would be directed to become T cells. They were wrong.
“We thought the cells present in the mouse that had never sensed the [Notch] signal … would wake up and become T cells once we allowed RBPJ to be expressed,” Zúñiga-Pflücker says. “But they didn’t.”
After learning that the road to becoming a T cell is paved prior to the thymus, the researchers dug deeper. Given that TSPs are made in the bone marrow, all signs pointed the team to there. Chen used an advanced technique called single-cell RNA sequencing to home in on TSPs, known to be a rare population of cells. Once they isolated the TSPs from a larger ‘cloud’ of cells, he switched Notch on in some, but not others. The ones where Notch was activated appeared to resemble cells on their way to becoming T cells. Not so for the other group.
“Looking within TSPs, which are defined by a certain set of genes, but in the absence of Notch signaling, they were not on the T cell path,” says Chen, who defended his PhD thesis in May 2019. Instead, they leaned toward becoming cells that give rise to macrophages, or as Zúñiga-Pflücker says, “a completely different lineage.”
Obtaining a more comprehensive understanding of T cell development could allow researchers to engineer them better for the treatment of immunodeficiency diseases. Thompson says better T cell therapies could help to combat tumours or be used to improve bone marrow transplants, too. Further, the mouse model created by the team has given scientists an essential new tool.
“The mouse model I created can be applied to study the effects of Notch signaling on any cell or tissue type,” Thompson says. Getting specific, Zúñiga-Pflücker notes that tissues in the brain, heart, kidneys, lungs, liver, skin and breasts stand to be investigated. “We’ll keep taking advantage of this mouse model to ask related questions,” he says.
Contemplating their results, Chen, who is now a postdoctoral fellow in Zúñiga-Pflücker’s lab, is satisfied to have brought some clarity to the origin of T cells. “It’s a question that’s been controversial for many years,” he says. “It’s a question a lot of people, a lot of groups, have been trying to answer.”
Zúñiga-Pflücker chimes in, too. “Nobody ever knew exactly what the true nature of a cell that leaves the marrow and goes to the thymus is. That’s been a quest for a long time—to find that cell that goes from one environment to another, and to find what makes it go there.”
With the early phase of T cell development now better understood, the lab will focus on the events that unfold after a T cell leaves the thymus. “Now we need to look at the later stages,” Zúñiga-Pflücker says.
Reflecting on the work, Thompson says she is thrilled. “It is great to finally be able to publish these findings in such a great journal. When I embarked on designing and generating this mouse model over 10 years ago, I didn’t necessarily think it would prove to be this impactful. I thought, at maximum, we would be answering a small question about T cell development that has been a point of contention in the field; however, with recent developments in immunotherapy that specifically involve T cells, this discovery has a much broader reach and application,” she says.
Chen is equally happy to share the findings. Alluding to the year-and-a-half editing process, he also says he’s relieved to release the paper, his first as lead author.
For now, though, T cells can wait. On the trio’s radar is celebrating over bowls of ramen.
Read text-only version of above infographic
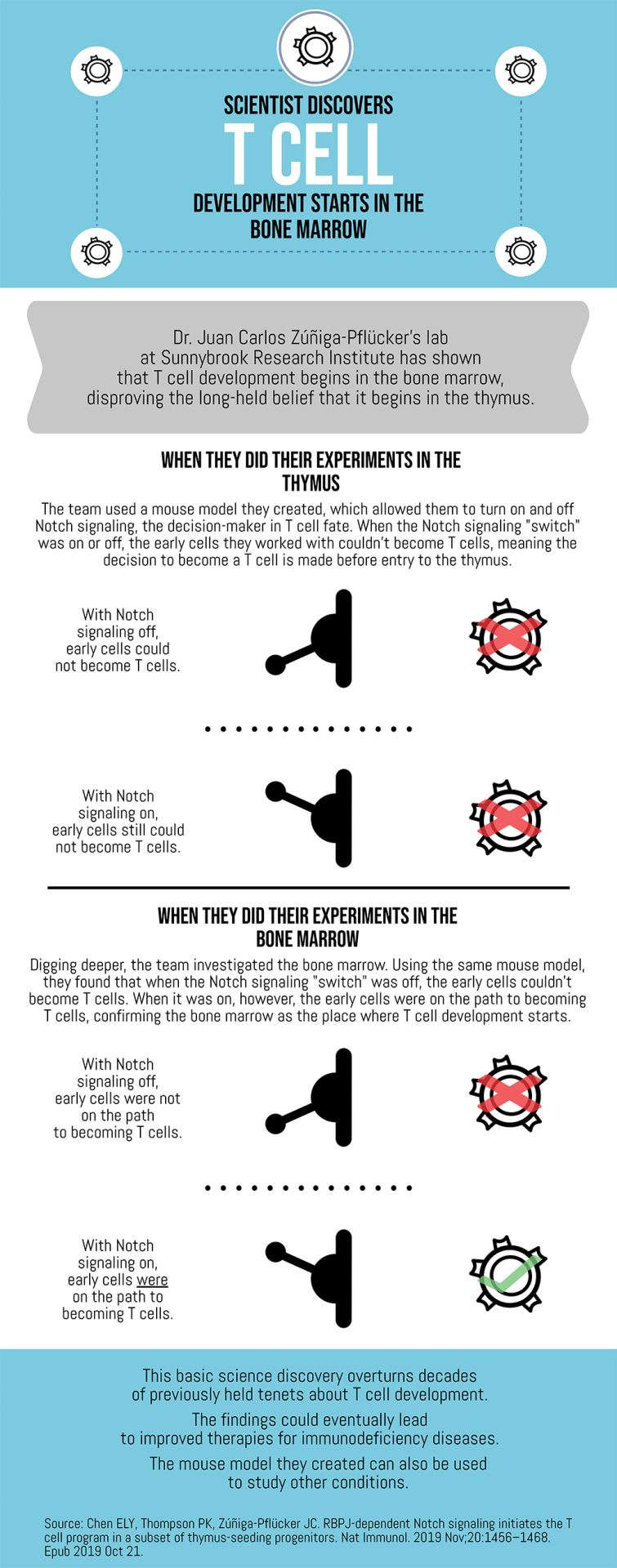
Scientist discovers T cell development starts in the bone marrow
Dr. Juan Carlos Zúñiga-Pflücker’s lab at Sunnybrook Research Institute has shown that T cell development begins in the bone marrow, disproving the long-held belief that it begins in the thymus.
-When they did their experiments in the thymus
The team used a mouse model they created, which allowed them to turn on and off Notch signaling, the decision-maker in T cell fate. When the Notch signaling “switch” was on or off, the early cells they worked with couldn’t become T cells, meaning the decision to become a T cell is made before entry to the thymus.
With Notch signaling off, early cells could not become T cells.
With Notch signaling on, early cells still could not become T cells.
-When they did their experiments in the bone marrow
Digging deeper, the team investigated the bone marrow. Using the same mouse model, they found that when the Notch signaling “switch” was off, the early cells couldn’t become T cells. When it was on, however, the early cells were on the path to becoming T cells, confirming the bone marrow as the place where T cell development starts.
With Notch signaling off, early cells were not on the path to becoming T cells.
With Notch signaling on, early cells were on the path to becoming T cells.
This basic science discovery overturns decades of previously held tenets about T cell development.
The findings could eventually lead to improved therapies for immunodeficiency diseases.
The mouse model they created can also be used to study other conditions.
Source: Chen ELY, Thompson PK, Zúñiga-Pflücker JC. RBPJ-dependent Notch signaling initiates the T cell program in a subset of thymus-seeding progenitors. Nat Immunol. 2019 Nov;20:1456–1468. Epub 2019 Oct 21.
This work was funded by grants from the Canadian Institutes of Health Research, the Krembil Foundation and National Institutes of Health to Zúñiga-Pflücker. Chen was supported by an Ontario Graduate Scholarship, and Thompson was supported by a Banting & Best Doctoral Research Award. Zúñiga-Pflücker was also supported by the Canada Research Chair in Developmental Immunology. His lab is part of the Centre for Research in Image-Guided Therapeutics at SRI, which was funded by the Canada Foundation for Innovation.
Original article: Chen ELY, Thompson PK, Zúñiga-Pflücker JC. RBPJ-dependent Notch signaling initiates the T cell program in a subset of thymus-seeding progenitors. Nat Immunol. 2019 Nov;20:1456–1468. Epub 2019 Oct 21.