Gene therapy targets the root cause of Parkinson’s disease noninvasively
A Toronto research team has demonstrated a noninvasive treatment strategy for Parkinson’s disease. In a world first, they used focused ultrasound—which applies sonic energy to target tissue deep in the brain or body—to deliver a therapeutic to specific brain regions in preclinical models of Parkinson’s disease.
Their target: alpha-synuclein, a protein that forms clumps in the brains of people with the disease that, over time, destroys neurons. The researchers used focused ultrasound to open the blood-brain barrier safely, and dispatch gene therapy. Doing so blocked production of the lethal protein by 50%.
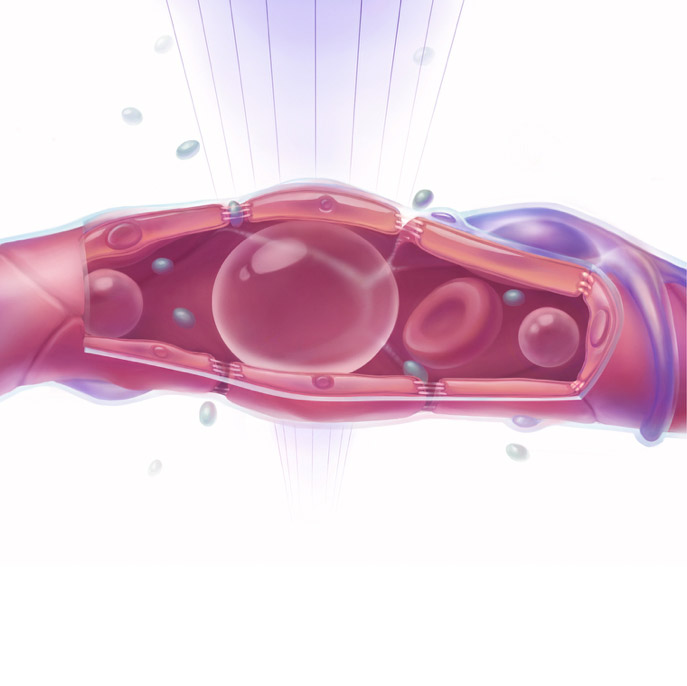
The study was published in Movement Disorders and highlighted in an editorial in the journal’s October 2018 issue. An image depicting the treatment that was created by members of the Aubert lab was chosen as the cover art of this issue.
“The synuclein protein, when it’s misfolded, can actually move from one cell to another, and this is the way that it may spread to different regions. There is a possibility that if you reduce the amount of synuclein in a healthy cell, you might be able to slow down the spreading pathology in a Parkinson brain,” says Dr. Anurag Tandon, a scientist at the Tanz Centre for Research in Neurodegenerative Diseases at the University of Toronto, and co-senior author of the study.
Parkinson’s disease is a neurodegenerative disorder that afflicts about 5 million people worldwide, including roughly 100,000 Canadians. It, like other brain disorders, is hard to treat because of the blood-brain barrier, which, as its name suggests, acts as a blockade between the brain’s capillaries and tissue. It prevents harmful substances in the blood from reaching the organ, but also blocks 98% of therapies from getting in.
To bypass this barrier and treat the root cause of Parkinson’s disease, for example, therapy would need to be injected directly into the brain—an invasive option since doctors would need to drill a hole in the skull. Complications include bleeding, infection and inadvertent damage to healthy brain cells. “To have a nonsurgical approach to gene therapy [for Parkinson’s disease] is very important, especially for elderly patients because it’s much easier on them. It can result in the same benefit, but without the potential complications that a surgery can have,” says Dr. Isabelle Aubert, a senior scientist in Biological Sciences at Sunnybrook Research Institute (SRI) and the study’s other co-senior author.
Gradually robbing people of the ability to control movement, Parkinson’s disease has no cure. Treatments are instead aimed at reducing symptoms like rigidity, shaking, and problems with balance and coordination.
Aubert and Tandon worked with Dr. Kullervo Hynynen, director of Physical Sciences at SRI and a co-author of the study who pioneered focused ultrasound. Hynynen was the first to combine MRI and focused ultrasound, and then to show that this combination could be used to open the blood-brain barrier safely, thereby inventing a new paradigm of treating diseases. Some years later, he and Aubert were the first to show preclinically that antibodies delivered with MRI-guided focused ultrasound can reduce amyloid in Alzheimer's disease. Those results are what prompted the researchers to study use of the technology in Parkinson’s disease.
The technique involves injecting tiny, harmless gas bubbles into the bloodstream. Next, a virus carrying the therapeutic payload—here, a gene sequence that blocks expression of alpha-synuclein—is inserted into the bloodstream. When the bubbles reach target areas, as shown on MRI, they are hit with focused ultrasound, causing them to expand and contract. This action causes the tight junctions that form the blood-brain barrier to loosen temporarily, allowing the therapeutic agent to slip through.
“The viral vector really gives the potential to have the gene integrated into the brain, and the therapeutic will be produced over and over again. It gives you the long, sustainable production of any therapeutic that you want,” says Aubert, noting that that in a Phase 1 clinical trial for Alzheimer’s disease, a single dose of a viral vector has been shown to deliver gene therapy for up to 10 years. In that study, the therapy was injected directly into the brain through small holes drilled through the skull.
An analysis of mice treated once with focused ultrasound and gene therapy showed that the expression of alpha-synuclein was about one-half of that in the untreated mice. Moreover, the treatment did not cause harm. “We didn’t see increased cell death or inflammatory changes like microglial or astroglial markers that were changed. That was comforting because we realized that we’re not doing damage to the brain regions that are being targeted with the focused ultrasound and biovector,” says Tandon.
Next, they will study whether the treatment improves motor function. “We’ve shown, as a first step, therapeutic efficacy at the molecular and cellular level. Now we need to show functional outcome—can we use it in an animal model that has motor deficits, and can we rescue those motor deficits?” says Aubert.
The co-first authors of this proof-of-principle study are Kristiana Xhima and Fadl Nabbouh, graduate students in the Aubert and Tandon labs, respectively. The research is early stage, but promising, given that those living with the disease, while being able to manage their symptoms, have no way of stopping its progression. Tandon says that more work needs to be done to improve the gene vectors, and the ability to target the therapy to different types of brain cells, but that he is optimistic about this approach. “People can live with Parkinson’s [disease] for many, many years. The downside is that regardless of how the symptoms are alleviated, the disease continues, and eventually they will succumb to the inability to move. I’m excited that we see the effect we were hoping for: a knockdown in synuclein expression. We know when we do that, we can prevent spreading pathology.”
This research was supported by the Canadian Institutes of Health Research and Weston Brain Institute. Dr. Hynynen is funded by the Canada Research Chairs Program and the U.S. National Institutes of Health. The Canada Foundation for Innovation provided infrastructure support through the Centre for Research in Image-Guided Therapeutics.