A model to mimic
It’s an extraordinary feat when a therapy to treat a disease as pervasive as advanced cancer proves even marginally successful in humans. The exorbitant costs, the efforts of specialists and the years of work are unfathomable, at least to people removed from the process. It’s a rare occurrence, too, which is a thorn in the side of Dr. Robert Kerbel, a senior scientist in Biological Sciences at Sunnybrook Research Institute (SRI).
Kerbel, who is also a professor in medical biophysics at the University of Toronto, has spent years exploring why studies so often yield positive results at the preclinical stage, but fall flat when they reach clinical trials, millions of dollars later. In his latest paper published in the British Journal of Cancer, he argues that the issue partly rests in how preclinical research is performed.
“What the [paper] shows is that there can be differences depending on whether we undertake treatment of a solitary primary tumour, or whether we do treatment where the primary tumour has been removed and we’re treating microscopic metastatic disease or overt metastatic disease,” Kerbel says. Metastatic disease is where cancer has spread from where it started, the primary site, to somewhere else in the body, such as the lungs, liver, brain or bone. In his view, potential therapies should be tested preclinically on various models of metastatic disease to predict better if a clinical trial of patients with similar disease that has spread will succeed. This is pivotal, and should be done before forking over large sums of money and possibly exposing people to harmful side effects, he argues.
“The vast majority of clinical trials in cancer patients are initially done on patients with advanced metastatic disease,” Kerbel says. Due to this, he stresses the need and rationale to test potential treatments preclinically in metastatic disease circumstances as well as earlier-stage disease scenarios, including only primary tumours.
To prove his point, Kerbel researched two forms of therapy, independently and with one another, on preclinical models. Some of these models had primary tumours, whereas others had cancer that had spread. This preclinical study design is uncommon, he says.
Typically in preclinical cancer research, tumour cells grown in a petri dish or extracted from people with cancer are injected into mice. In the case of the latter, immune-suppressed mice are used. (These are mice whose immune systems have been essentially deactivated.) A tumour begins to develop, and once it has reached a certain size, researchers treat it with whatever therapy they’re exploring. “It’s fast, simple and relatively inexpensive,” Kerbel says.
The flaw with this method, Kerbel says, is that it may fail to address what happens when treating cancer that has spread. He says that this oversight is one reason mouse studies can flop when they’re replicated in human trials. “Traditionally, and to this day, most investigators do not assess the effects of their new drug or their new therapy on metastatic disease in mice,” he says. This could be due to lack of awareness or funds, or because preclinical research on metastatic disease is more cumbersome, he adds.
For his studies, Kerbel developed models where the primary tumour was not the only object of investigation. “We resected [cut out the tumour], allowed the mice to develop either early- or late-stage metastatic disease, and then we initiated the treatment.” The two treatments he investigated were immune checkpoint inhibitors and antiangiogenic agents.
Immune checkpoint inhibitors are drugs that boost and sustain the immune system to fight cancer cells in the body. Antiangiogenic therapies starve tumours by impairing their supply of blood vessels. By robbing them of this lifeline, the agents force the tumours to undergo the equivalent of a heart attack, Kerbel says.
Kerbel’s team delivered the treatments and looked for differences in response. “We noted that there were circumstances where something worked in the primary tumour treatment setting but didn’t work so well, if at all, in the [metastatic disease] setting,” he says.
In contrast, to illuminate the variety of results that can be gleaned when testing therapies on different models, he discusses his studies on paclitaxel, a commonly used drug to treat metastatic breast cancer patients. Here, when paclitaxel was used alone, it had a benefit in the metastatic setting, but not in the primary tumour model.
The principal purpose of Kerbel’s recent paper, he says, is to show that differences in results, sometimes significant, do arise in studies on various stages of disease. These should be taken into account prior to initiating expensive human trials involving thousands of cancer patients. “We’re not saying you should abandon the traditional approach—that’s a starting point—but if you obtain a positive result with the primary tumour model, maybe before embarking on these multimillion dollar clinical trials, try to refine and extend your results by evaluating the kind of treatment situation you’re going to be assessing in the clinic,” he says.
Kerbel continues: “The take-home message is that yes, do the primary tumour treatment model studies, but supplement them, when possible, with these other models that deal with the treatment of either early- or late-stage metastatic disease, to get a better idea, hopefully, of how things might play out when these drugs are tested in cancer patients that have similar stages of metastatic disease.”
In the case of many preclinical studies where new drugs are being developed, reaching the clinic is a distant aim. The opposite is true for Kerbel’s work. “This is not something that’s way off in the future. What we’re doing is taking drugs that are clinically approved—we’re not in the business of developing new drugs—and asking how you might use them and test them, especially in combination. We’re utilizing these newer, more rigorous models that will hopefully be more accurate in telling clinical investigators, ‘Yes, they’re going to have a higher probability of working; or no, it’s unlikely that they’ll work,’” he says. Theoretically, if an encouraging result emerges from a preclinical study that includes work on metastatic disease, then a more informed decision can be made about subsequent clinical development, he says.
Although Kerbel says most groups continue to concentrate their research on primary tumours as opposed to metastatic disease, he underscores the unexpected findings that can result from a shift in approach: “You can find out all sorts of interesting things that you wouldn’t necessarily discover if you restricted your studies to only treating primary tumours.”
Reflecting on his career, Kerbel says, “Evaluating therapies in a different way than is traditionally done, this emphasis on metastasis, that’s been the thread of my research for almost four decades.” Placing his most recent project—and the studies that paved the way to it—in his canon of findings, he adds, “I think the importance will be its potential impact on getting other preclinical cancer researchers working in the burgeoning field of cancer immunotherapy to consider proactively adopting similar approaches centered on treatment of metastatic disease.”
Read text-only version of above infographic
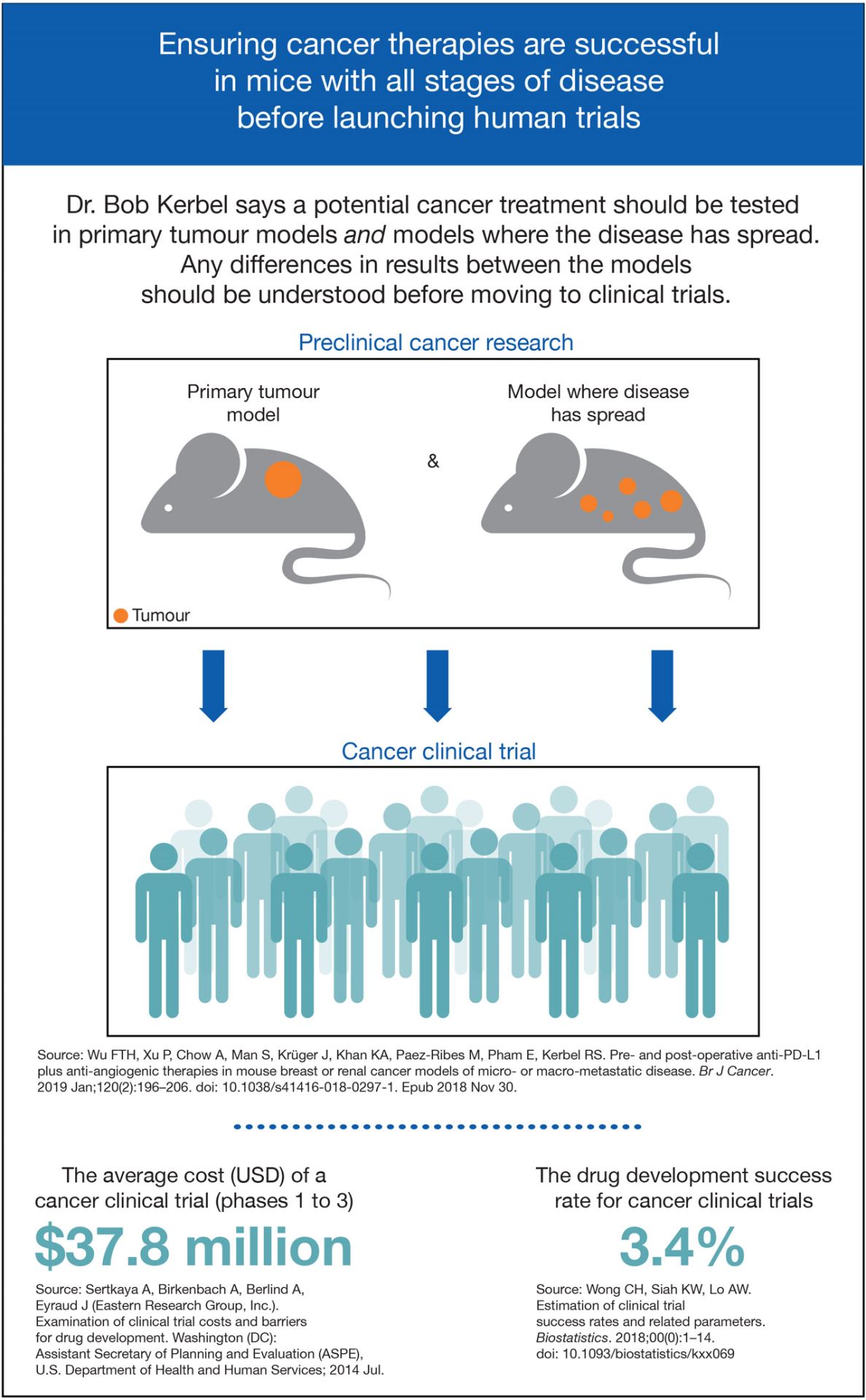
Ensuring cancer therapies are successful in mice with all stages of disease before launching human trials
Dr. Bob Kerbel says a potential cancer treatment should be tested in primary tumour models and models where the disease has spread. Any differences in results between the models should be understood before moving to clinical trials.
Preclinical cancer research
- Primary tumour model
- Model where disease has spread
Cancer clinical trials
The average cost (USD) of a cancer clinical trial (phases 1 to 3): $37.8 million
The drug development success rate for cancer clinical trials: 3.4%
Sources:
Wu FTH, Xu P, Chow A, Man S, Krüger J, Khan KA, Paez-Ribes M, Pham E, Kerbel RS. Pre- and post-operative anti-PD-L1 plus anti-angiogenic therapies in mouse breast or renal cancer models of micro- or macro-metastatic disease. Br J Cancer. 2019 Jan;120(2):196–206. doi: 10.1038/s41416-018-0297-1. Epub 2018 Nov 30.
Sertkaya A, Birkenbach A, Berlind A, Eyraud J (Eastern Research Group, Inc.). Examination of clinical trial costs and barriers for drug development. Washington (DC): Assistant Secretary of Planning and Evaluation (ASPE), U.S. Department of Health and Human Services; 2014 Jul.
Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2018;00(0):1–14. doi: 10.1093/biostatistics/kxx069
This work was funded by the Canadian Breast Cancer Foundation, the Canadian Institutes of Health Research and Worldwide Cancer Research. The Canada Foundation for Innovation provided infrastructure support through the Centre for Research in Image-Guided Therapeutics.
Original article: Wu FTH, Xu P, Chow A, Man S, Krüger J, Khan KA, Paez-Ribes M, Pham E, Kerbel RS. Pre- and post-operative anti-PD-L1 plus anti-angiogenic therapies in mouse breast or renal cancer models of micro- or macro-metastatic disease. Br J Cancer. 2019 Jan;120(2):196–206. doi: 10.1038/s41416-018-0297-1. Epub 2018 Nov 30.
In a nutshell
- Dr. Robert Kerbel has shown that significant differences in results can surface when testing a therapy on a mouse whose cancer exists in one tumour versus a mouse whose cancer has spread to other areas.
- He did tests on mice using various drugs, individually and in combination, to treat multiple forms of cancer.
- The findings emphasize the need to study such differences before spending vast amounts of money on clinical trials and potentially putting the safety of participants at risk.