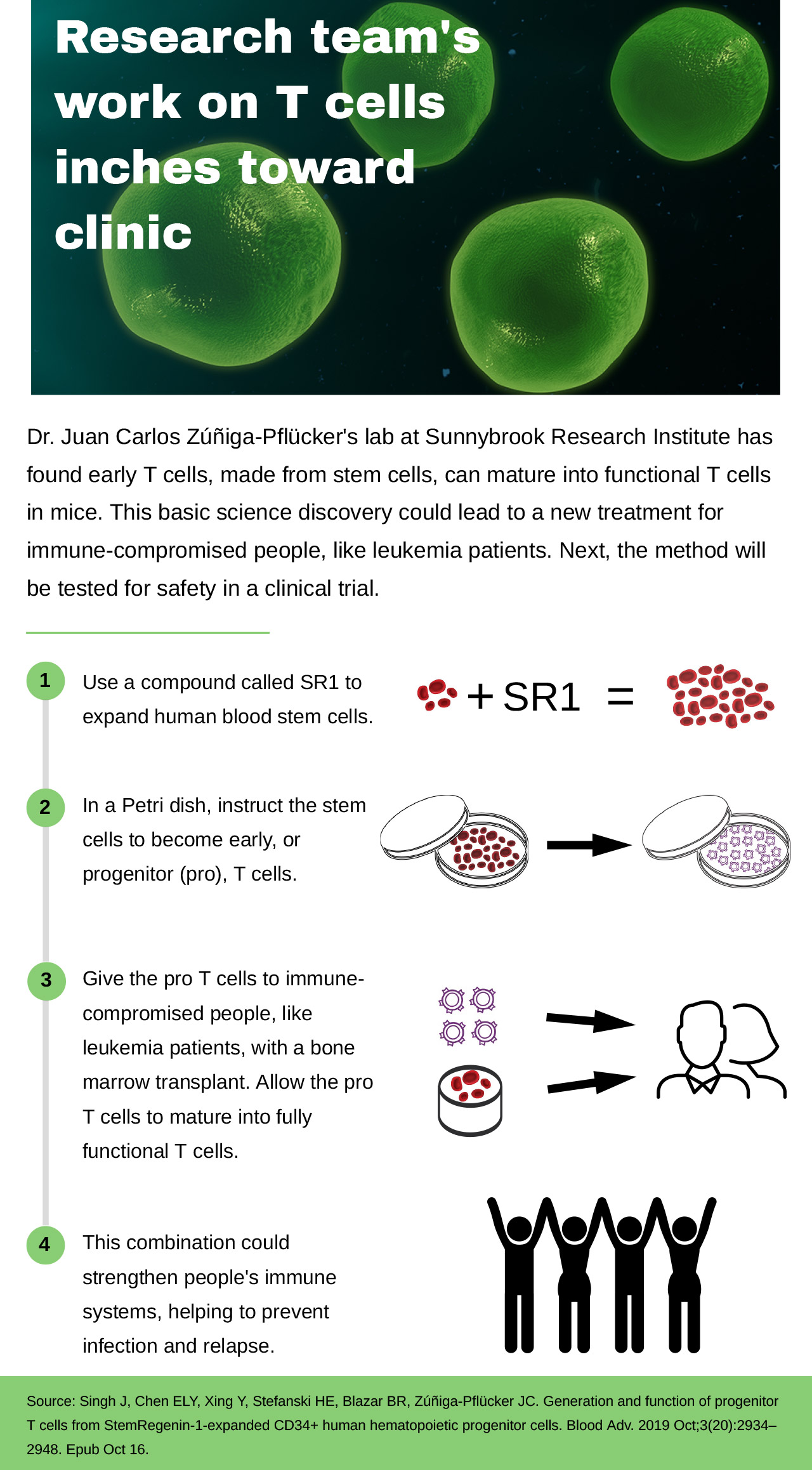
Research team's work on T cells inches toward clinic
It was around 2014 when Dr. Jasty Singh says she and her lab mates at Sunnybrook Research Institute (SRI) began thinking about generating mass amounts of stem cells to use in their experiments. Blood stem cells are useful because they can be directed to become any type of cell. A potential clinical application was far from their minds, though—it was basic research with which they were concerned. Fast-forward a handful of years, however, and Singh’s work is on the cusp of clinical testing.
Singh, who completed her PhD in the lab of Dr. Juan Carlos Zúñiga-Pflücker, a senior scientist in Biological Sciences at SRI, is part of a group that has shown it is possible to make early T cells from human blood stem cells in a Petri dish, deliver them to an immune-compromised mouse and then allow those early T cells to develop into mature T cells.
Published in the October 2019 edition of Blood Advances, this work on T cells—white blood cells that are vital to healthy immunity—could offer people with weakened immune systems, owing to diseases like leukemia, for example, new hope. It’s also the product of collaboration. The SRI lab, headed by Zúñiga-Pflücker and including Singh and postdoctoral fellow Dr. Edward Chen, partnered with a team led by Drs. Bruce Blazar and Heather Stefanski at the University of Minnesota.
“We’ve made use of a new chemical compound, StemRegenin-1, or SR1, which allows hematopoietic stem cells—the source of all bloods cells—to be grown into a larger number of cells while retaining their blood stem cell properties,” says Zúñiga-Pflücker, who is also the chair of and a professor in the department of immunology at the University of Toronto. “We then instructed these cells to become progenitor [or early] T cells, which have the ability to home to the thymus and mature into fully functioning T cells.”
The efforts began when it was discovered that blood stem cells could be expanded with SR1 to make progenitor (pro) T cells. The issue, as Singh and her lab mates realized about five years ago, was the scarcity of human blood stem cells. Singh explains this is because there is a lack of cord blood donors, and blood and bone marrow, from which stem cells can be isolated, do not contain enough of these cells.
“We make pro T cells in the lab all the time, but the source—stem cells—is so rare that we can never make enough,” says Singh, who is now an assistant professor, teaching stream, in the department of immunology at U of T. “[With SR1], we now have a way to make a limitless source of stem cells, then make them into pro T cells, and then characterize those pro T cells.”
The team wondered whether it was feasible for pro T cells made from SR1-expanded stem cells, via Zúñiga-Pflücker’s OP9-DL1 system, to be instructed to become functional T cells. The OP9-DL1 system was established almost two decades ago and is the premier method for developing T cells in the lab. The answer to the team’s query was yes.
“What we show in the paper is that the pro T cells we made not only look like the pro T cells we’ve studied for so long, based on cell surface markers, but they also behave like them. When we put them in mice, they engrafted in the thymus. When we developed them into mature T cells, they functionally responded—they secreted a bunch of chemical signals that are important for host defence,” Singh says. “We did genetic analyses showing that there are all these different types of T cells that can be generated, which would be normally expected from a pro T cell.”
For people with deficient immune systems, this could be a lifesaver. Singh explains that leukemia patients might be among the first to benefit. When a person with this type of cancer is treated, they often receive chemotherapy. Although these treatments kill cancer cells, they also wipe out good cells, leaving a person’s immune system ravaged. To restore a healthy cell population, bone marrow transplantation, or a stem cell transplant, is given; T cells are slow to rebuild, however, meaning a person is left even more vulnerable to infection and relapse.
“Complementing a stem cell transplant with pro T cells would really help accelerate the response, meaning that the patient wouldn’t be immunodeficient for a long period after the transplant. It would increase the survival of the patient,” Singh says.
The focus now is to make the protocol compliant with good manufacturing practices, a job already underway in Minnesota. This means ensuring every stage of the process satisfies the government’s most stringent standards for use in humans. If approved, then it’s full steam ahead toward a Phase 1 clinical trial to test safety and feasibility, which the researchers say could begin in one year.
“The next steps will be primarily carried out by the Minnesota team, but they will rely on our scientific guidance as they move forward,” Zúñiga-Pflücker says.
Considering the work, Zúñiga-Pflücker turns to the process of developing a lab-based discovery into a possible treatment. “I am delighted that our initial work on the basic principles of how T cells develop in the thymus, and our fundamental scientific discovery of the critical molecular underpinning events that control the generation of T cells, can now be fully translated into new clinical therapies that will benefit patients in need of new T cells.”
Singh says she is excited to see a project of hers approach the clinic, but it’s the journey to this point from which she draws joy. “The ability to work with a multidisciplinary team has been a unique learning experience and a growth opportunity,” she says. “One of the things I appreciated about this work is that it was highly collaborative. There are three main PIs [principal investigators] involved, and they all have very different sources of expertise. I think that’s what allowed this to be fruitful and to become a project in the first place.”
Read text-only version of above infographic
Research team’s work on T cells inches toward clinic
Dr. Juan Carlos Zúñiga-Pflücker’s lab at Sunnybrook Research Institute has found early T cells, made from stem cells, can mature into functional T cells in mice. This basic science discovery could lead to a new treatment for immune-compromised people, like leukemia patients. Next, the method will be tested for safety in a clinical trial.
- Use a compound called SR1 to expand human blood stem cells.
- In a Petri dish, instruct the stem cells to become early, or progenitor (pro), T cells.
- Give the pro T cells to immune-compromised people, like leukemia patients, with a bone marrow transplant. Allow the pro T cells to mature into fully functional T cells.
- This combination could strengthen people’s immune systems, helping to prevent infection and relapse.
Source: Singh J, Chen ELY, Xing Y, Stefanski HE, Blazar BR, Zúñiga-Pflücker JC. Generation and function of progenitor T cells from StemRegenin-1-expanded CD34+ human hematopoietic progenitor cells. Blood Adv. 2019 Oct;3(20):2934–2948. Epub Oct 16.
This work was supported by the Canadian Cancer Society, Canadian Institutes of Health Research, Children’s Cancer Research Fund, Krembil Foundation, National Institutes of Health, Ontario Institute for Regenerative Medicine, St. Baldrick’s Foundation and University of Toronto. Zúñiga-Pflücker was also supported by the Canada Research Chair in Developmental Immunology. His lab is part of the Centre for Research in Image-Guided Therapeutics at SRI, which was funded by the Canada Foundation for Innovation.
Original article: Singh J, Chen ELY, Xing Y, Stefanski HE, Blazar BR, Zúñiga-Pflücker JC. Generation and function of progenitor T cells from StemRegenin-1-expanded CD34+ human hematopoietic progenitor cells. Blood Adv. 2019 Oct;3(20):2934–2948. Epub Oct 16.
In a nutshell
- A Sunnybrook Research Institute trio has discovered that progenitor (pro) T cells, made from stem cells, can mature into functional T cells in mice.
- These pro T cells could potentially be coupled with bone marrow transplantation to treat immune-compromised people, like leukemia patients.
- The protocol is being made compatible with good manufacturing practices, with the aim of launching a clinical trial in one year.